Drug Catalog - Product Detail
ZOLMITRIPTAN 5MG TAB 3X1 UD
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65862-0915-64 | AUROBINDO PHARMA | 3 | 5MG | NA |
PACKAGE FILES
Generic Name
ZOLMITRIPTAN
Substance Name
ZOLMITRIPTAN
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA207021
Description
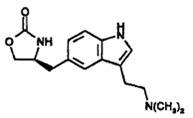
11 DESCRIPTION Zolmitriptan tablets, USP contain zolmitriptan, which is a selective 5-hydroxytryptamine 1B/1D (5-HT 1B/1D ) receptor agonist. Zolmitriptan is chemically designated as (S)-4-[[3-[2-(dimethylamino)ethyl]-1H-indol-5-yl]methyl]-2-oxazolidinone and has the following chemical structure: The molecular formula is C 16 H 21 N 3 O 2 , representing a molecular weight of 287.36. Zolmitriptan is a white to off-white powder that is readily soluble in water. Zolmitriptan tablets, USP are available as 2.5 mg (yellow and functionally-score) and 5 mg (pink, not scored) film-coated tablets for oral administration. The film-coated tablets contain anhydrous lactose, colloidal silicon dioxide, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, sodium starch glycolate, and titanium dioxide. In addition, the 2.5 mg tablets contain yellow iron oxide and the 5 mg tablets contain red iron oxide. Zolmitriptan Chemical Structure
How Supplied
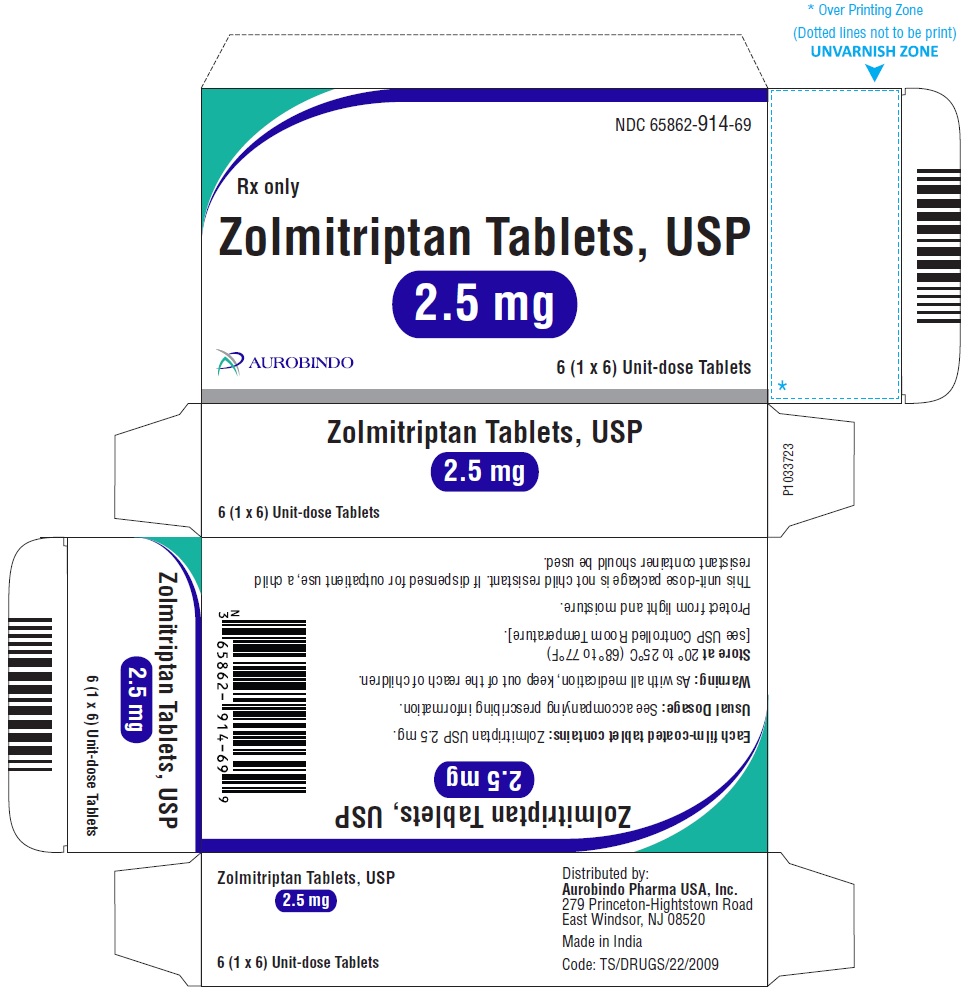
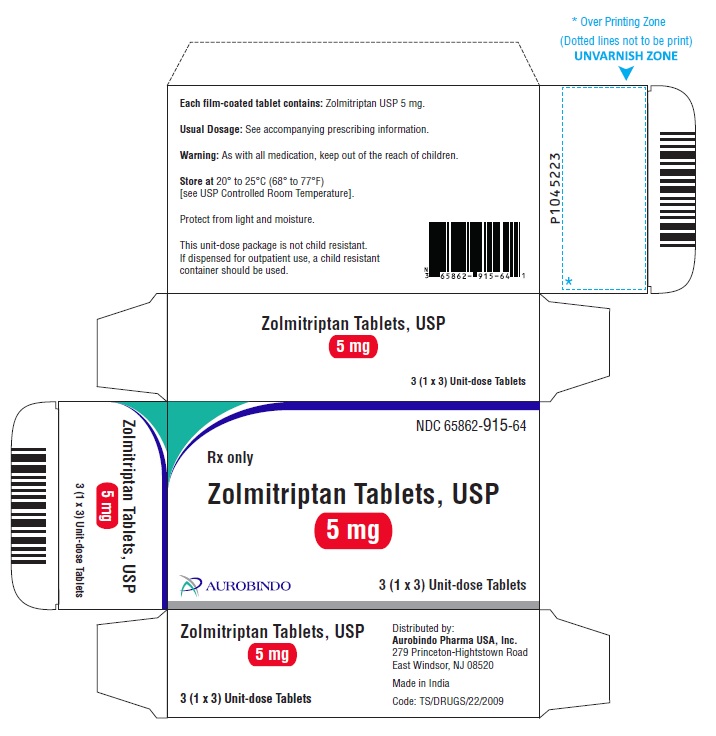
16 HOW SUPPLIED/STORAGE AND HANDLING Zolmitriptan Tablets, USP 2.5 mg are yellow colored, round, biconvex, film-coated functionally-scored tablets debossed with ‘C’ and ‘C’ on either side of the score line on one side and ‘37’ on the other side. Carton of 6 (1 x 6) Unit-dose Tablets NDC 65862-914-69 Zolmitriptan Tablets, USP 5 mg are pink colored, round, biconvex, film-coated tablets debossed with ‘CC’ on one side and ‘51’ on the other side. Carton of 3 (1 x 3) Unit-dose Tablets NDC 65862-915-64 Store zolmitriptan tablets at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from light and moisture.
Indications & Usage
1 INDICATIONS AND USAGE Zolmitriptan tablets are indicated for the acute treatment of migraine with or without aura in adults. Limitations of Use Only use zolmitriptan tablets if a clear diagnosis of migraine has been established. If a patient has no response to zolmitriptan tablets treatment for the first migraine attack, reconsider the diagnosis of migraine before zolmitriptan tablets are administered to treat any subsequent attacks. Zolmitriptan tablets are not indicated for the prevention of migraine attacks. Safety and effectiveness of zolmitriptan tablets have not been established for cluster headache. Zolmitriptan tablets are a serotonin (5-HT) 1B/1D receptor agonist (triptan) indicated for the acute treatment of migraine with or without aura in adults (1) Limitations of Use: Use only after a clear diagnosis of migraine has been established (1) Not indicated for the prophylactic therapy of migraine (1) Not indicated for the treatment of cluster headache (1)
Dosage and Administration
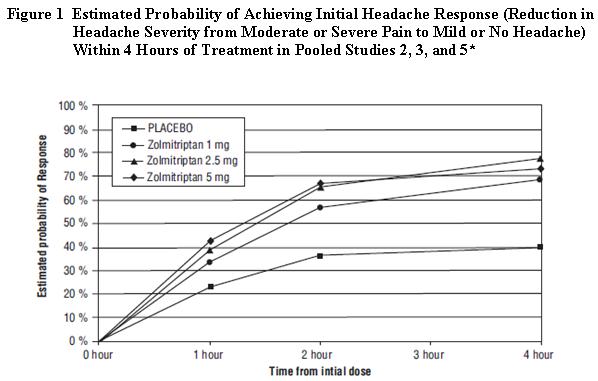
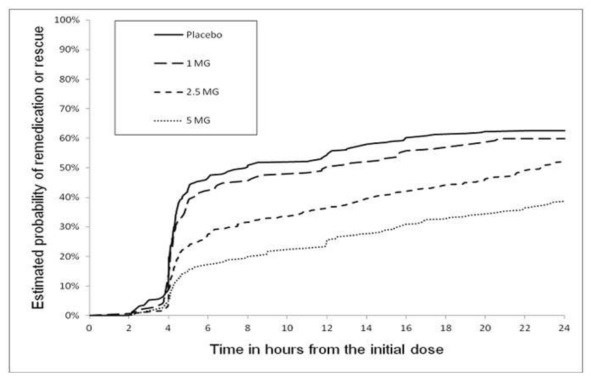
2 DOSAGE AND ADMINISTRATION Recommended starting dose: 1.25 mg or 2.5 mg (2.1) Maximum single dose: 5 mg (2.1) May repeat dose after 2 hours if needed; not to exceed 10 mg in any 24-hour period (2.1) Moderate or Severe Hepatic Impairment: 1.25 mg recommended (2.3 , 8.6) 2.1 Dosing Information The recommended starting dose of zolmitriptan tablets is 1.25 mg or 2.5 mg. The 1.25 mg dose can be achieved by manually breaking the functionally-scored 2.5 mg tablet in half. The maximum recommended single dose of zolmitriptan tablets is 5 mg. In controlled clinical trials, a greater proportion of patients had headache response following a 2.5 mg or 5 mg dose than following a 1 mg dose. There was little added benefit from the 5 mg dose compared to the 2.5 mg dose, but adverse reactions were more frequent with the 5 mg dose. If the migraine has not resolved by 2 hours after taking zolmitriptan tablets, or returns after a transient improvement, a second dose may be administered at least 2 hours after the first dose. The maximum daily dose is 10 mg in any 24-hour period. The safety of zolmitriptan tablets in the treatment of an average of more than three migraines in a 30-day period has not been established. 2.3 Dosing in Patients with Hepatic Impairment The recommended dose of zolmitriptan tablets in patients with moderate to severe hepatic impairment is 1.25 mg (one-half of one 2.5 mg zolmitriptan tablet) because of increased zolmitriptan blood levels in these patients and elevation of blood pressure in some of these patients. Limit the total daily dose in patients with severe hepatic impairment to no more than 5 mg per day. 2.4 Dosing in Patients taking Cimetidine If zolmitriptan tablets are co-administered with cimetidine, limit the maximum single dose of zolmitriptan tablets to 2.5 mg, not to exceed 5 mg in any 24-hour period [see Drug Interactions (7.5) , Clinical Pharmacology (12.3) ] .
