Drug Catalog - Product Detail
ZIPRASIDONE HCL CP 20MG 60
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
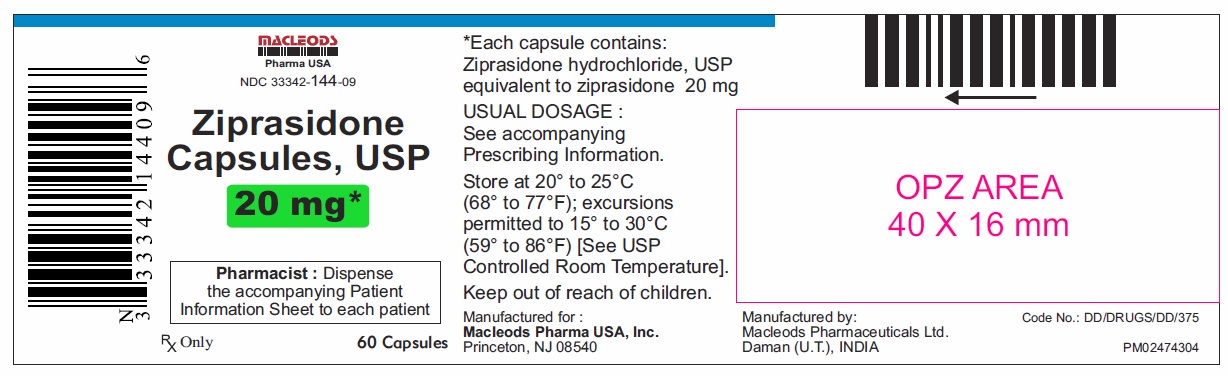
| 33342-0144-09 | MACLEODS PHARMACEUTICALS | 60 | 20MG | CAPSULE |
PACKAGE FILES








Generic Name
ZIPRASIDONE HYDROCHLORIDE
Substance Name
ZIPRASIDONE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA204375
Description
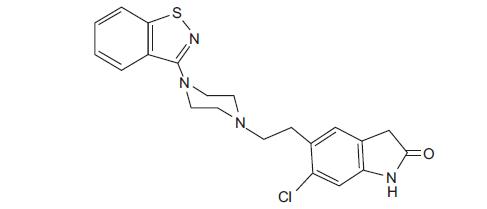
11 DESCRIPTION Ziprasidone capsules, USP contains the active moiety, ziprasidone, in the form of ziprasidone hydrochloride salt. Ziprasidone is a psychotropic agent that is chemically unrelated to phenothiazine or butyrophenone antipsychotic agents. It has a molecular weight of 412.94 (free base), with the following chemical name: 5-[2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]ethyl]-6-chloro-1,3-dihydro- 2H -indol-2-one. The empirical formula of C 21 H 21 ClN 4 OS (free base of ziprasidone) represents the following structural formula: Ziprasidone capsules, USP contain a monohydrochloride, monohydrate salt of ziprasidone. Chemically, ziprasidone hydrochloride monohydrate is 5-[2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]ethyl]-6-chloro-1,3-dihydro- 2H -indol-2-one, monohydrochloride, monohydrate. The empirical formula is C 21 H 21 ClN 4 OS • HCl • H 2 O and its molecular weight is 467.42. Ziprasidone hydrochloride monohydrate is a white to slightly pink powder. Ziprasidone capsules, USP are supplied for oral administration in 20 mg (blue/white), 40 mg (blue/blue), 60 mg (white/white), and 80 mg (blue/white) capsules. Ziprasidone capsules, USP contain ziprasidone hydrochloride USP, lactose monohydrate, polyethylene glycol, sodium starch glycolate, ammonium chloride, sucrose and sodium lauryl sulfate. Each capsule for oral use contains ziprasidone hydrochloride monohydrate equivalent to either 20 mg, 40 mg, 60 mg, or 80 mg of ziprasidone. str
How Supplied
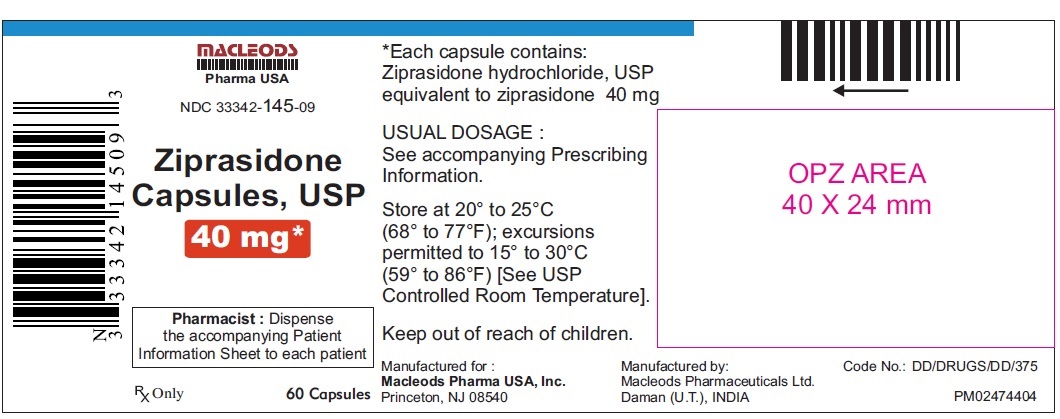
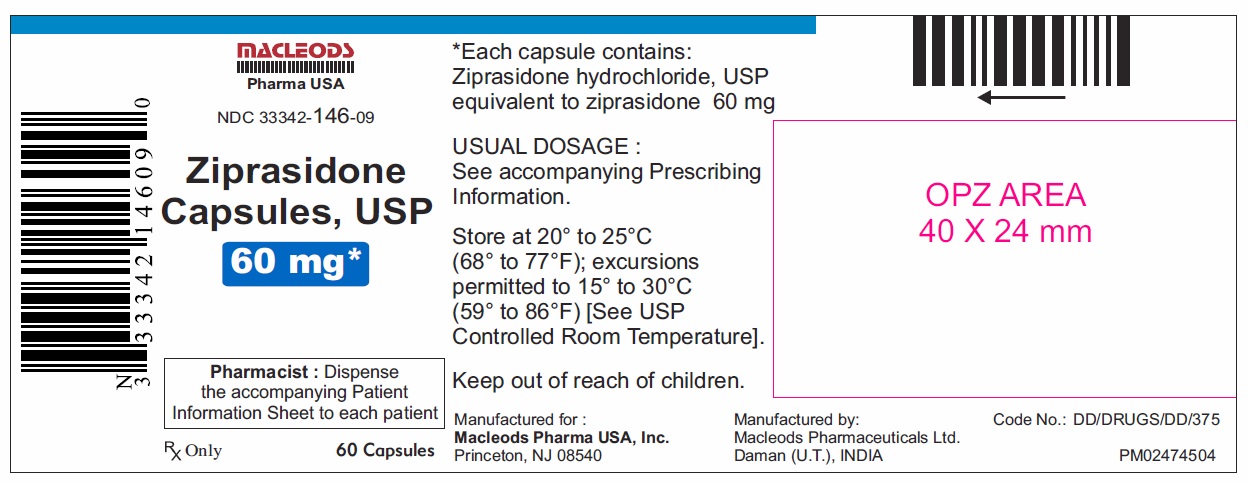
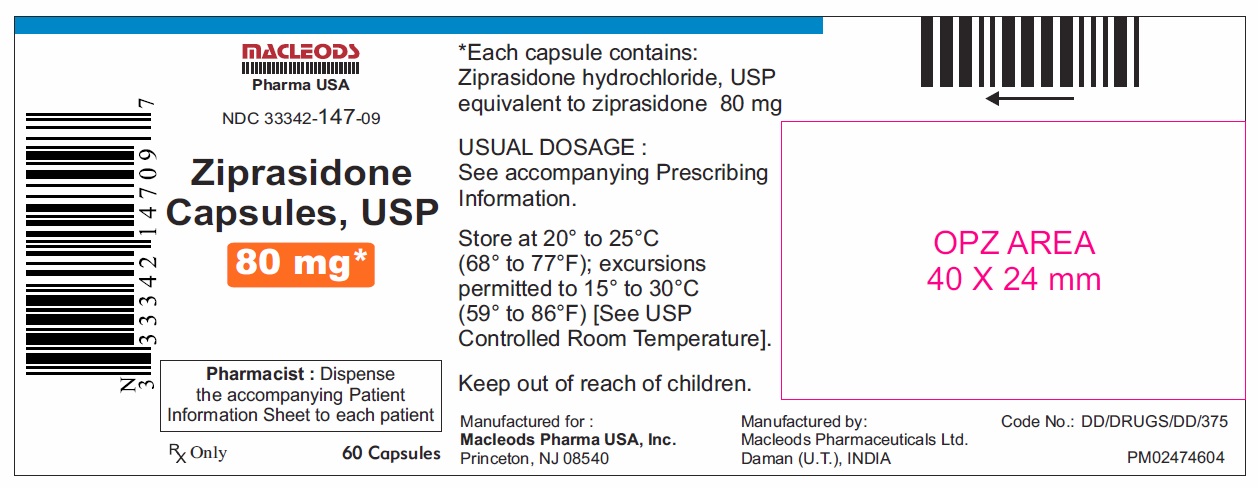
16 HOW SUPPLIED/STORAGE AND HANDLING Ziprasidone capsules, USP 20 mg are blue/white, size 4, hard gelatin capsules imprinted in black ink with “CL62” on cap and “20 mg” on the body containing pale pink coloured powder and are available as follows: Bottles of 60 capsules NDC 33342-144-09 Bottles of 300 capsules NDC 33342-144-51 Unit dose blister pack of 100 NDC 33342-144-12 (10×10) capsules Ziprasidone capsules, USP 40 mg are blue/ blue, size 2, hard gelatin capsules imprinted in black ink with “CL63” on cap and “40 mg” on the body containing pale pink coloured powder and are available as follows: Bottles of 60 capsules NDC 33342-145-09 Bottles of 300 capsules NDC 33342-145-51 Unit dose blister pack of 100 NDC 33342-145-12 (10×10) capsules Ziprasidone capsules, USP 60 mg are white/ white, size 1, hard gelatin capsules imprinted in black ink with “CL64” on cap and “60 mg” on the body containing pale pink coloured powder and are available as follows: Bottles of 60 capsules NDC 33342-146-09 Bottles of 300 capsules NDC 33342-146-51 Unit dose blister pack of 100 NDC 33342-146-12 (10×10) capsules Ziprasidone capsules, USP 80 mg are blue/ white, size 0, hard gelatin capsules imprinted in black ink with “CL65” on cap and “80 mg” on the body containing pale pink coloured powder and are available as follows: Bottles of 60 capsules NDC 33342-147-09 Bottles of 300 capsules NDC 33342-147-51 Unit dose blister pack of 100 NDC 33342-147-12 (10×10) capsules Store ziprasidone capsules, USP at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Preserve in well-closed containers, and store at controlled room temperature.
Indications & Usage
1 INDICATIONS & USAGE Ziprasidone capsules are indicated for the treatment of schizophrenia, as monotherapy for the acute treatment of bipolar manic or mixed episodes, and as an adjunct to lithium or valproate for the maintenance treatment of bipolar disorder. When deciding among the alternative treatments available for the condition needing treatment, the prescriber should consider the finding of ziprasidone’s greater capacity to prolong the QT/QTc interval compared to several other antipsychotic drugs [ see Warnings and Precautions ( 5.3 ) ]. Prolongation of the QTc interval is associated in some other drugs with the ability to cause torsade de pointes-type arrhythmia, a potentially fatal polymorphic ventricular tachycardia, and sudden death. In many cases this would lead to the conclusion that other drugs should be tried first. Whether ziprasidone will cause torsade de pointes or increase the rate of sudden death is not yet known [ see Warnings and Precautions ( 5.3 ) ] Schizophrenia • Ziprasidone capsules are indicated for the treatment of schizophrenia in adults [ see Clinical Studies ( 14.1 ) ]. Bipolar I Disorder (Acute Mixed or Manic Episodes and Maintenance Treatment as an Adjunct to Lithium or Valproate) • Ziprasidone capsules are indicated as monotherapy for the acute treatment of adults with manic or mixed episodes associated with bipolar I disorder [ see Clinical Studies ( 14.2 ) ]. • Ziprasidone capsules are indicated as an adjunct to lithium or valproate for the maintenance treatment of bipolar I disorder in adults [ see Clinical Studies ( 14.2 ) ]. Ziprasidone hydrochloride is an atypical antipsychotic. In choosing among treatments, prescribers should be aware of the capacity of ziprasidone hydrochloride to prolong the QT interval and may consider the use of other drugs first ( 1 ) Ziprasidone capsules are indicated for the: • treatment of schizophrenia in adults. ( 1 ) • acute treatment of adults as monotherapy of manic or mixed episodes associated with bipolar I disorder. ( 1 ) • maintenance treatment of bipolar I disorder as an adjunct to lithium or valproate in adults. ( 1 )
Dosage and Administration
2 DOSAGE & ADMINISTRATION Administer capsules orally with food. Do not open, crush, or chew. ( 2.1 ) Schizophrenia: Initiate at 20 mg twice daily. Daily dosage may be adjusted up to 80 mg twice daily. Dose adjustments should occur at intervals of not less than 2 days. Safety and efficacy has been demonstrated in doses up to 100 mg twice daily. The lowest effective dose should be used. ( 2.2 ) Acute treatment of manic/mixed episodes of bipolar I disorder: Initiate at 40 mg twice daily. Increase to 60 mg or 80 mg twice daily on day 2 of treatment. Subsequent dose adjustments should be based on tolerability and efficacy within the range of 40-80 mg twice daily. ( 2.3 ) Maintenance treatment of bipolar I disorder as an adjunct to lithium or valproate: Continue treatment at the same dose on which the patient was initially stabilized, within the range of 40-80 mg twice daily. ( 2.3 ) 2.1 Administration Information for Ziprasidone Capsules Administer ziprasidone capsules orally with food. Swallow capsules whole, do not open, crush, or chew the capsules. 2.2 Schizophrenia Dose Selection Ziprasidone capsules should be administered at an initial daily dose of 20 mg twice daily with food. In some patients, daily dosage may subsequently be adjusted on the basis of individual clinical status up to 80 mg twice daily. Dosage adjustments, if indicated, should generally occur at intervals of not less than 2 days, as steady-state is achieved within 1 to 3 days. In order to ensure use of the lowest effective dose, patients should ordinarily be observed for improvement for several weeks before upward dosage adjustment. Efficacy in schizophrenia was demonstrated in a dose range of 20 mg to 100 mg twice daily in short-term, placebo-controlled clinical trials. There were trends toward dose response within the range of 20 mg to 80 mg twice daily, but results were not consistent. An increase to a dose greater than 80 mg twice daily is not generally recommended. The safety of doses above 100 mg twice daily has not been systematically evaluated in clinical trials [see Clinical Studies ( 14.1 )]. Maintenance Treatment While there is no body of evidence available to answer the question of how long a patient treated with ziprasidone should remain on it, a maintenance study in patients who had been symptomatically stable and then randomized to continue ziprasidone or switch to placebo demonstrated a delay in time to relapse for patients receiving ziprasidone capsule [see Clinical Studies ( 14.1 )]. No additional benefit was demonstrated for doses above 20 mg twice daily. Patients should be periodically reassessed to determine the need for maintenance treatment. 2.3 Bipolar I Disorder (Acute Mixed or Manic Episodes and Maintenance Treatment as an Adjunct to Lithium or Valproate) Acute Treatment of Manic or Mixed Episodes In adults oral ziprasidone should be administered at an initial daily dose of 40 mg twice daily with food. The dose may then be increased to 60 mg or 80 mg twice daily on the second day of treatment and subsequently adjusted on the basis of tolerance and efficacy within the range 40 mg-80 mg twice daily. In the flexible-dose clinical trials, the mean daily dose administered was approximately 120 mg [ see Clinical Studies ( 14.2 ) ]. Maintenance Treatment (as an adjunct to lithium or valproate) Continue treatment at the same dose on which the patient was initially stabilized, within the range of 40 mg-80 mg twice daily with food. Patients should be periodically reassessed to determine the need for maintenance treatment [ see Clinical Studies ( 14.2 ) ].
