Drug Catalog - Product Detail
ZIDOVUDINE TB 300MG 60
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 31722-0509-60 | CAMBER PHARMACEUTICALS | 60 | 300MG | TABLET |
PACKAGE FILES

Generic Name
ZIDOVUDINE
Substance Name
ZIDOVUDINE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA090092
Description
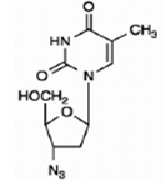
11 DESCRIPTION Zidovudine (formerly called azidothymidine [AZT]), a pyrimidine nucleoside analogue active against HIV-1. The chemical name of zidovudine is 3'- azido-3'-deoxythymidine; it has the following structural formula: Zidovudine USP is a white to beige, odorless, crystalline solid with a molecular weight of 267.24 and a solubility of 20.1 mg per mL in water at 25°C. The molecular formula is C 10 H 13 N 5 O 4 . Zidovudine tablets, USP are for oral administration. Each film-coated tablet contains 300 mg of zidovudine USP and the inactive ingredients microcrystalline cellulose, sodium starch glycolate, magnesium stearate and opadry white. (hypromellose, polyethylene glycol, and titanium dioxide). ZidovudineStructure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Zidovudine Tablets USP, 300 mg are white to off white colored, biconvex, round film coated tablets debossed with 'H' on one side and '1' on other side. Bottle of 60 (NDC 31722-509-60). Store at 20° to 25° C (68° to 77°F) [see USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS & USAGE Zidovudine tablets are a nucleoside analogue reverse transcriptase inhibitor indicated for: •Treatment of Human Immunodeficiency Virus (HIV-1) infection in combination with other antiretroviral agents. ( 1.1 ) • Prevention of maternal-fetal HIV-1 transmission. ( 1.2 ) 1.1 Treatment of HIV-1 Zidovudine tablets, a nucleoside reverse transcriptase inhibitor, are indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection. 1.2 Prevention of Maternal-Fetal HIV-1 Transmission Zidovudine tablets are indicated for the prevention of maternal-fetal HIV-1 transmission [see Dosage and Administration ( 2.3 )]. The indication is based on a dosing regimen that included 3 components: 1. antepartum therapy of HIV-1-infected mothers 2. intrapartum therapy of HIV-1-infected mothers 3. post-partum therapy of HIV-1-exposed neonate Points to consider prior to initiating zidovudine in pregnant women for the prevention of maternal-fetal HIV-1 transmission include: • In most cases, zidovudine for prevention of maternal-fetal HIV-1 transmission should be given in combination with other antiretroviral drugs. • Prevention of HIV-1 transmission in women who have received zidovudine for a prolonged period before pregnancy has not been evaluated. • Because the fetus is most susceptible to the potential teratogenic effects of drugs during the first 10 weeks of gestation and the risks of therapy with zidovudine during that period are not fully known, women in the first trimester of pregnancy who do not require immediate initiation of antiretroviral therapy for their own health may consider delaying use; this indication is based on use after 14 weeks' gestation.
Dosage and Administration
2 DOSAGE & ADMINISTRATION • Treatment of HIV-1 infection: Adults: Recommended oral dosage is 300 mg twice a day with other antiretroviral agents. ( 2.1 ) Pediatric patients (aged 4 weeks to less than 18 years): Dosage should be calculated based on body weight not to exceed adult dose. ( 2.2 ) • Prevention of maternal-fetal HIV-1 transmission: Specific dosage instructions for mother and infant. ( 2.3 ) • Patients with severe anemia and/or neutropenia: Dosage interruption may be necessary. ( 2.4 ) • Renal impairment: Recommended oral dosage in hemodialysis or peritoneal dialysis or in patients with creatinine clearance (CrCl) less than mL per minute is 100 mg every 6 to 8 hours. ( 2.5 ) 2.1 Adults - Treatment of HIV-1 Infection Oral Dosing The recommended oral dose of zidovudine is 300 mg twice daily in combination with other antiretroviral agents. 2.2 Pediatric Patients (Aged 4 Weeks to Less than 18 Years) Healthcare professionals should pay special attention to accurate calculation of the dose of zidovudine, transcription of the medication order, dispensing information, and dosing instructions to minimize risk for medication dosing errors. Prescribers should calculate the appropriate dose of zidovudine for each child based on body weight (kg) and should not exceed the recommended adult dose. Before prescribing zidovudine tablets, children should be assessed for the ability to swallow tablets. If a child is unable to reliably swallow a zidovudine tablet, the zidovudine syrup formulation should be prescribed. The recommended oral dosage in pediatric patients aged 4 weeks to less than 18 years and weighing greater than or equal to 4 kg is provided in Table 1. Zidovudine Syrup should be used to provide accurate dosage when tablets are not appropriate. Table 1. Recommended Pediatric Oral Dosage of Zidovudine Body Weight (kg) Total Daily Dose Dosage Regimen and Dose Twice Daily Three Times Daily 4 to <9 24 mg/kg/day 12 mg/kg 8 mg/kg ≥9 to <30 18 mg/kg/day 9 mg/kg 6 mg/kg ≥30 600 mg/day 300 mg 200 mg Alternatively, dosing for zidovudine can be based on body surface area (BSA) for each child. The recommended oral dose of zidovudine is 480 mg per m 2 per day in divided doses (240 mg per m2 twice daily or 160 mg per m 2 three times daily). In some cases the dose calculated by mg per kg will not be the same as that calculated by BSA. 2.3 Prevention of Maternal-Fetal HIV-1 Transmission The recommended dosage regimen for administration to pregnant women (greater than 14 weeks of pregnancy) and their neonates is: Maternal Dosing 100 mg orally 5 times per day until the start of labor [see Clinical Studies ( 14.3 )]. During labor and delivery, intravenous zidovudine should be administered at 2 mg per kg (total body weight) over 1 hour followed by a continuous intravenous infusion of 1 mg per kg per hour (total body weight) until clamping of the umbilical cord. Neonatal Dosing Start neonatal dosing within 12 hours after birth and continue through 6 weeks of age. Neonates unable to receive oral dosing may be administered zidovudine intravenously. See Table 2 for dosing recommendations. Table 2. Recommended Neonatal Dosages of Zidovudine Route Total Daily Dose Dose and Dosage Regimen Oral 8 mg/kg/day 2 mg/kg every 6 hours Intravenous 6 mg/kg/day 1.5 mg/kg infused over 30 minutes, every 6 hours Use an appropriate-sized syringe with 0.1-mL graduation to ensure accurate dosing of the syrup formulation in neonates. 2.4 Patients with Severe Anemia and/or Neutropenia Significant anemia (hemoglobin less than 7.5 g per dL or reduction greater than 25% of baseline) and/or significant neutropenia (granulocyte count less than 750 cells per mm 3 or reduction greater than 50% from baseline) may require a dose interruption until evidence of marrow recovery is observed [see Warnings and Precautions ( 5.1 )]. In patients who develop significant anemia, dose interruption does not necessarily eliminate the need for transfusion. If marrow recovery occurs following dose interruption, resumption in dose may be appropriate using adjunctive measures such as epoetin alfa at recommended doses, depending on hematologic indices such as serum erythropoietin level and patient tolerance. 2.5 Patients with Renal Impairment In patients maintained on hemodialysis or peritoneal dialysis or with creatinine clearance (CrCl) by Cockcroft-Gault less than 15 mL per min, the recommended oral dosage is 100 mg every 6 to 8 hours [see Use in Specific Populations ( 8.6 ), Clinical Pharmacology ( 12.3 )]. 2.6 Patients with Hepatic Impairment There are insufficient data to recommend dose adjustment of zidovudine in patients with impaired hepatic function or liver cirrhosis. Frequent monitoring of hematologic toxicities is advised [see Use in Specific Populations ( 8.7 )].
