Drug Catalog - Product Detail
ZAFIRLUKAST TB 20MG 60
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 49884-0554-02 | PAR PHARMACEUTICAL | 60 | 20MG | TABLET |
PACKAGE FILES


Generic Name
ZAFIRLUKAST
Substance Name
ZAFIRLUKAST
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
NDA020547
Description
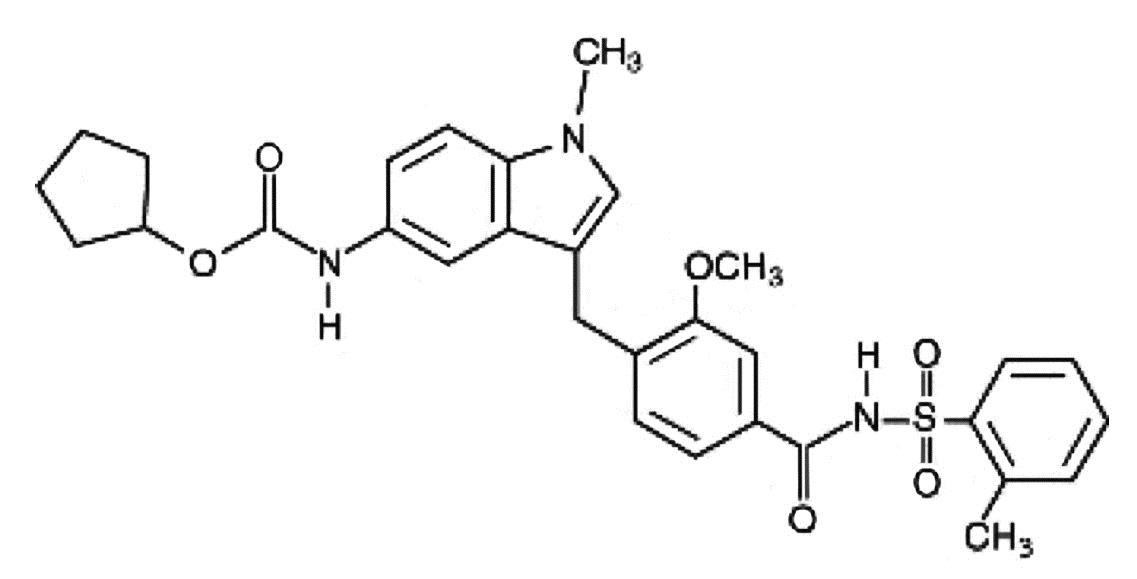
DESCRIPTION Zafirlukast is a synthetic, selective peptide leukotriene receptor antagonist (LTRA), with the chemical name 4-(5-cyclopentyloxy-carbonylamino-1-methyl-indol-3-ylmethyl)-3-methoxy-N-o-tolylsulfonylbenzamide. The molecular weight of zafirlukast is 575.7 and the structural formula is: The empirical formula is: C 31 H 33 N 3 O 6 S Zafirlukast, a fine white to pale yellow amorphous powder, is practically insoluble in water. It is slightly soluble in methanol and freely soluble in tetrahydrofuran, dimethylsulfoxide, and acetone. Zafirlukast is supplied as 10 and 20 mg tablets for oral administration. Inactive Ingredients: Film-coated tablets containing croscarmellose sodium, lactose, magnesium stearate, microcrystalline cellulose, povidone, hypromellose, and titanium dioxide. structure
How Supplied
HOW SUPPLIED Zafirlukast 10 mg Tablets, (NDC 49884-549-02) white, round, biconvex, film-coated tablets debossed with “P” on one side and “10” on the other, are supplied in opaque HDPE bottles of 60 tablets. Zafirlukast 20 mg Tablets, (NDC 49884-554-02) white, round, biconvex, film-coated tablets debossed with “P” on one side and “20” on the other, are supplied in opaque HDPE bottles of 60 tablets. Store at controlled room temperature, 20-25°C (68-77°F) [see USP]. Protect from light and moisture. Dispense in the original air-tight container.
Indications & Usage
INDICATIONS AND USAGE Zafirlukast is indicated for the prophylaxis and chronic treatment of asthma in adults and children 5 years of age and older.
Dosage and Administration
DOSAGE AND ADMINISTRATION Because food can reduce the bioavailability of zafirlukast, zafirlukast should be taken at least 1 hour before or 2 hours after meals. Adults and Children 12 years of age and older The recommended dose of zafirlukast in adults and children 12 years and older is 20 mg twice daily. Pediatric Patients 5 through 11 years of age The recommended dose of zafirlukast in children 5 through 11 years of age is 10 mg twice daily. Elderly Patients Based on cross-study comparisons, the clearance of zafirlukast is reduced in elderly patients (65 years of age and older), such that C max and AUC are approximately twice those of younger adults. In clinical trials, a dose of 20 mg twice daily was not associated with an increase in the overall incidence of adverse events or withdrawals because of adverse events in elderly patients. Patients with Hepatic Impairment Zafirlukast is contraindicated in patients with hepatic impairment including hepatic cirrhosis (see Contraindications). The clearance of zafirlukast is reduced in patients with stable alcoholic cirrhosis such that the C max and AUC are approximately 50 - 60% greater than those of normal adults. Zafirlukast has not been evaluated in patients with hepatitis or in long-term studies of patients with cirrhosis. Patients with Renal Impairment Dosage adjustment is not required for patients with renal impairment.
