Drug Catalog - Product Detail
Wixela Inhub 100-50mcg/dose Aerosol Powder Breath Activated
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00378-9320-32 | MYLAN | 60 | 100-50MCG/ACT | AEROSOL |
PACKAGE FILES











Generic Name
FLUTICASONE PROPIONATE AND SALMETEROL
Substance Name
FLUTICASONE PROPIONATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
RESPIRATORY (INHALATION)
Application Number
ANDA208891
Description
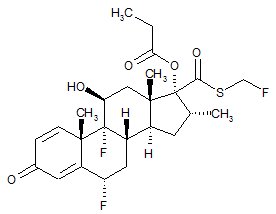
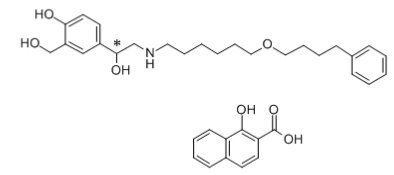
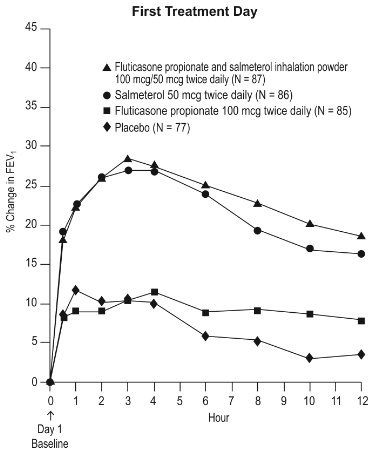
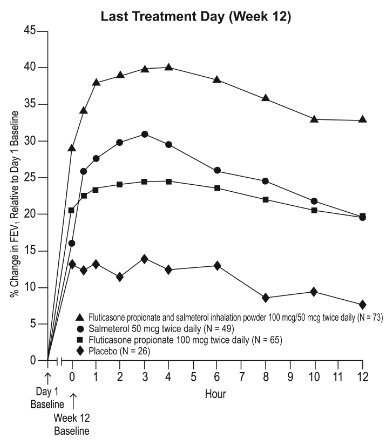
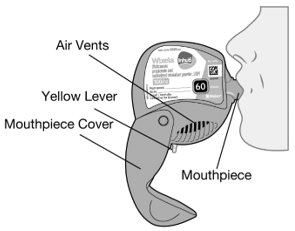
11 DESCRIPTION Wixela Inhub ® 100/50, Wixela Inhub ® 250/50, and Wixela Inhub ® 500/50 are combinations of fluticasone propionate and salmeterol xinafoate. One active component of Wixela Inhub ® is fluticasone propionate, a corticosteroid having the chemical name S -Fluoromethyl 6α,9α-difluoro-11β-hydroxy-16α-methyl-3-oxo-17α-propionyloxyandrosta-1,4-diene-17β-carbothioate and the following chemical structure: Fluticasone propionate, USP is a white to almost white powder with a molecular weight of 500.6, and the empirical formula is C 25 H 31 F 3 O 5 S. It is practically insoluble in water, freely soluble in dimethyl sulfoxide and dimethylformamide, and slightly soluble in methanol and 95% ethanol. The other active component of Wixela Inhub ® is salmeterol xinafoate, a beta 2 -adrenergic bronchodilator. Salmeterol xinafoate is the racemic form of the 1-hydroxy-2-naphthoic acid salt of salmeterol. It has the chemical name (±)-4-Hydroxy-α 1 -[[[6-(4-phenylbutoxy)hexyl]amino]methyl]- m -xylene-α,α'-diol 1-hydroxy-2-naphthoate (salt) and the following chemical structure: Salmeterol xinafoate, USP is a white to almost white powder with a molecular weight of 603.8, and the empirical formula is C 25 H 37 NO 4 •C 11 H 8 O 3 . It is freely soluble in methanol; slightly soluble in ethanol, chloroform, and isopropanol; and sparingly soluble in water. Wixela Inhub ® is a grey colored plastic inhaler containing two foil sealed discs, each disc containing 30 pre-metered doses. Each of the 60 doses contains a white to off white powder mix of micronized fluticasone propionate (100, 250, or 500 mcg) and micronized salmeterol xinafoate salt (72.5 mcg, equivalent to 50 mcg of salmeterol base) in 12.5 mg of formulation containing lactose monohydrate (which contains milk proteins). After the inhaler is activated, the powder is dispersed into the airstream created by the patient inhaling through the mouthpiece. Under standardized in vitro test conditions, Wixela Inhub ® delivers 93, 233, and 465 mcg of fluticasone propionate and 45 mcg of salmeterol base per dose from Wixela Inhub ® 100/50, Wixela Inhub ® 250/50, and Wixela Inhub ® 500/50, respectively, when tested at a flow rate of 60 L/min for 2 seconds. In adult subjects with obstructive lung disease and severely compromised lung function (mean FEV 1 20% to 30% of predicted), mean peak inspiratory flow (PIF) through another dry powder inhaler was 82.4 L/min (range: 46.1 to 115.3 L/min). Inhalation profiles for adolescent (N = 13, aged 12 to 17 years) and adult (N = 17, aged 18 to 50 years) subjects with asthma inhaling maximally through another dry powder inhaler show mean PIF of 122.2 L/min (range: 81.6 to 152.1 L/min). Inhalation profiles for pediatric subjects with asthma inhaling maximally through another dry powder inhaler show a mean PIF of 75.5 L/min (range: 49.0 to 104.8 L/min) for the 4-year-old subject set (N = 20) and 107.3 L/min (range: 82.8 to 125.6 L/min) for the 8-year-old subject set (N = 20). The actual amount of drug delivered to the lung will depend on patient factors, such as inspiratory flow profile. Meets USP Aerodynamic Particle Size Distribution Test 2. Fluticasone Propionate Structural Formula Salmeterol Zinofoate Structural Formula
How Supplied
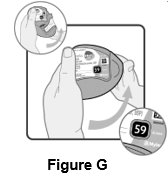
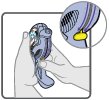
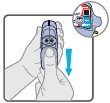
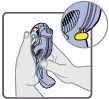
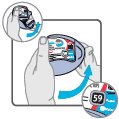
16 HOW SUPPLIED/STORAGE AND HANDLING Wixela Inhub ® 100/50 is supplied as a disposable grey colored plastic dry powder inhaler containing two foil sealed discs, providing a total of 60 pre-metered doses. The inhaler is packaged in a moisture-protective foil pouch. NDC 0378-9320-32 carton containing one dry powder inhaler Wixela Inhub ® 250/50 is supplied as a disposable grey colored plastic dry powder inhaler containing two foil sealed discs, providing a total of 60 pre-metered doses. The inhaler is packaged in a moisture-protective foil pouch. NDC 0378-9321-32 carton containing one dry powder inhaler Wixela Inhub ® 500/50 is supplied as a disposable grey colored plastic dry powder inhaler containing two foil sealed discs, providing a total of 60 pre-metered doses. The inhaler is packaged in a moisture-protective foil pouch. NDC 0378-9322-32 carton containing one dry powder inhaler Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.] Store in a dry place away from direct heat and sunlight. Keep this and all medication out of the reach of children. Wixela Inhub ® should be stored inside the unopened moisture-protective foil pouch and only removed from the pouch immediately before initial use. Discard Wixela Inhub ® 1 month after opening the foil pouch or when the counter reads “0” (after all doses have been used), whichever comes first. The inhaler is not reusable. Do not attempt to take the inhaler apart.
Indications & Usage
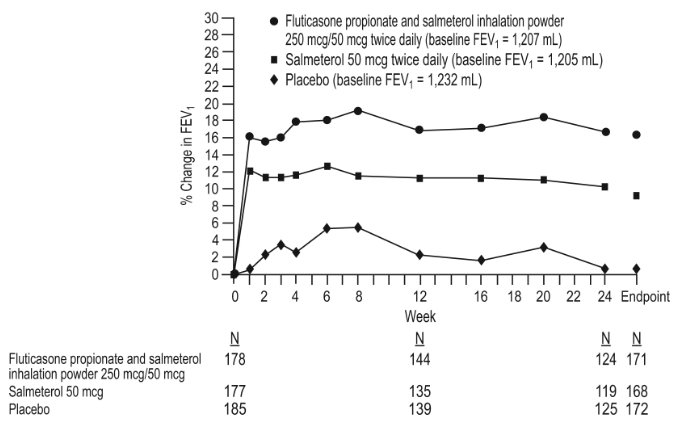
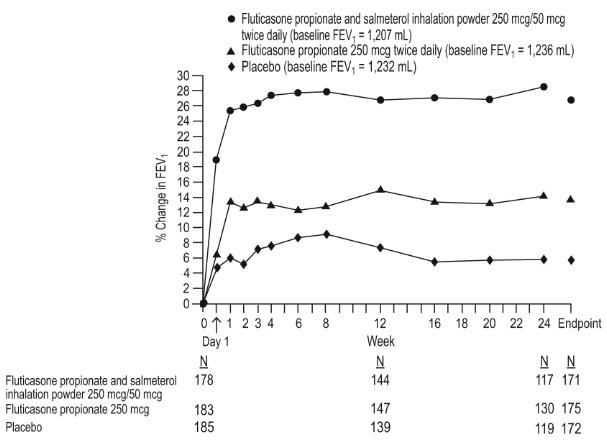
1 INDICATIONS AND USAGE Wixela Inhub ® is a combination product containing a corticosteroid and a long-acting beta 2 -adrenergic agonist (LABA) indicated for: • Twice-daily treatment of asthma in patients aged 4 years and older. ( 1.1 ) • Maintenance treatment of airflow obstruction and reducing exacerbations in patients with chronic obstructive pulmonary disease (COPD). ( 1.2 ) Important limitation of use: Not indicated for relief of acute bronchospasm. ( 1.1 , 1.2 ) 1.1 Treatment of Asthma Wixela Inhub ® is indicated for the twice-daily treatment of asthma in patients aged 4 years and older. Wixela Inhub ® should be used for patients not adequately controlled on a long-term asthma control medication such as an inhaled corticosteroid (ICS) or whose disease warrants initiation of treatment with both an ICS and long-acting beta 2 -adrenergic agonist (LABA). Important Limitation of Use Wixela Inhub ® is NOT indicated for the relief of acute bronchospasm. 1.2 Maintenance Treatment of Chronic Obstructive Pulmonary Disease Wixela Inhub ® 250/50 is indicated for the twice-daily maintenance treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema. Wixela Inhub ® 250/50 is also indicated to reduce exacerbations of COPD in patients with a history of exacerbations. Wixela Inhub ® 250/50 twice daily is the only approved dosage for the treatment of COPD because an efficacy advantage of the higher strength Wixela Inhub ® 500/50 over Wixela Inhub ® 250/50 has not been demonstrated. Important Limitation of Use Wixela Inhub ® is NOT indicated for the relief of acute bronchospasm.
Dosage and Administration
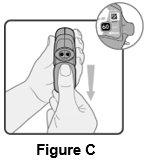
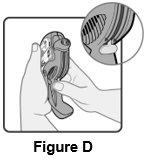
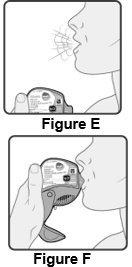
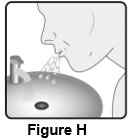
2 DOSAGE AND ADMINISTRATION Wixela Inhub ® should be administered as 1 inhalation twice daily by the orally inhaled route only. After inhalation, the patient should rinse his/her mouth with water without swallowing to help reduce the risk of oropharyngeal candidiasis. More frequent administration or a greater number of inhalations (more than 1 inhalation twice daily) of the prescribed strength of Wixela Inhub ® is not recommended as some patients are more likely to experience adverse effects with higher doses of salmeterol. Patients using Wixela Inhub ® should not use additional LABA for any reason. [See Warnings and Precautions (5.3 , 5.12) .] For oral inhalation only. ( 2 ) • Treatment of asthma in patients aged 12 years and older: 1 inhalation of Wixela Inhub ® 100/50, Wixela Inhub ® 250/50, or Wixela Inhub ® 500/50 twice daily. Starting dosage is based on asthma severity. ( 2.1 ) • Treatment of asthma in patients aged 4 to 11 years: 1 inhalation of Wixela Inhub ® 100/50 twice daily. ( 2.1 ) • Maintenance treatment of COPD: 1 inhalation of Wixela Inhub ® 250/50 twice daily. ( 2.2 ) 2.1 Asthma If asthma symptoms arise in the period between doses, an inhaled, short-acting beta 2 -agonist should be taken for immediate relief. Adult and Adolescent Patients Aged 12 Years and Older For patients aged 12 years and older, the dosage is 1 inhalation twice daily, approximately 12 hours apart. When choosing the starting dosage strength of Wixela Inhub ® , consider the patients’ disease severity, based on their previous asthma therapy, including the ICS dosage, as well as the patients’ current control of asthma symptoms and risk of future exacerbation. The maximum recommended dosage is Wixela Inhub ® 500/50 twice daily. Improvement in asthma control following inhaled administration of Wixela Inhub ® can occur within 30 minutes of beginning treatment, although maximum benefit may not be achieved for 1 week or longer after starting treatment. Individual patients will experience a variable time to onset and degree of symptom relief. For patients who do not respond adequately to the starting dosage after 2 weeks of therapy, replacing the current strength of Wixela Inhub ® with a higher strength may provide additional improvement in asthma control. If a previously effective dosage regimen fails to provide adequate improvement in asthma control, the therapeutic regimen should be reevaluated and additional therapeutic options (e.g., replacing the current strength of Wixela Inhub ® with a higher strength, adding additional ICS, initiating oral corticosteroids) should be considered. Pediatric Patients Aged 4 to 11 Years For patients with asthma aged 4 to 11 years who are not controlled on an ICS, the dosage is 1 inhalation of Wixela Inhub ® 100/50 twice daily, approximately 12 hours apart. 2.2 Chronic Obstructive Pulmonary Disease The recommended dosage for patients with COPD is 1 inhalation of Wixela Inhub ® 250/50 twice daily, approximately 12 hours apart. If shortness of breath occurs in the period between doses, an inhaled, short-acting beta 2- agonist should be taken for immediate relief.
