Drug Catalog - Product Detail
VALGANCICLOVIR TB 450MG 60
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 31722-0832-60 | CAMBER PHARMACEUTICALS | 60 | 450MG | TABLET |
PACKAGE FILES


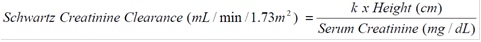

Generic Name
VALGANCICLOVIR
Substance Name
VALGANCICLOVIR HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA205166
Description
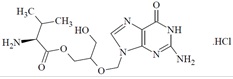
11 DESCRIPTION Valganciclovir tablets, USP contains valganciclovir hydrochloride, USP a hydrochloride salt of the L-valyl ester of ganciclovir that exists as a mixture of two diastereomers. Ganciclovir is a synthetic guanine derivative active against CMV. Valganciclovir tablets, USP are available as a 450 mg tablet for oral administration. Each film coated tablet contains 496.3 mg of valganciclovir hydrochloride, USP (corresponding to 450 mg of valganciclovir), and the inactive ingredients crospovidone, microcrystalline cellulose, povidone and stearic acid. The tablets are coated with Opadry Pink which contains hypromellose, iron oxide red, polyethylene glycol, polysorbate 80 and titanium dioxide. Valganciclovir hydrochloride, USP is a white to off-white powder, slightly hygroscopic with a molecular formula of C14H22N6O5•HCl and a molecular weight of 390.82. The chemical name for valganciclovir hydrochloride, USP is L-Valine, 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]-3-hydroxypropylester, monohydrochloride. Valganciclovir hydrochloride, USP is a polar hydrophilic compound with a solubility of 70 mg/mL in water at 25°C at a pH of 7 and an n-octanol/water partition coefficient of 0.0095 at pH 7. The pKa for valganciclovir hydrochloride, USP is 7.5. The chemical structure of valganciclovir hydrochloride, USP is: All doses in this insert are specified in terms of valganciclovir. valganstructure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Valganciclovir tablets USP, 450 mg are pink, oval, biconvex, film-coated tablets, debossed with 'J' on one side and '156' on the other side. Each film-coated tablet contains 496.3 mg of valganciclovir hydrochloride, USP equivalent to 450 mg of valganciclovir. Valganciclovir tablets are supplied in: Bottles of 60 tablets NDC 31722-832-60 Blister card of 10 Unit dose tablets NDC 31722-832-31 Blister pack of 100 (10 x 10) Unit dose tablets NDC 31722-832-32 Store at 25 o C; excursions permitted between 15 o and 30 o C (59 o and 86 o F). [See USP Controlled Room Temperature.]
Indications & Usage
1 INDICATIONS AND USAGE Valganciclovir tablets are a deoxynucleoside analogue cytomegalovirus (CMV) DNA polymerase inhibitor indicated for: Adult Patients ( 1.1 ) • Treatment of CMV retinitis in patients with acquired immunodeficiency syndrome (AIDS). • Prevention of CMV disease in kidney, heart, and kidney-pancreas transplant patients at high risk. Pediatric Patients ( 1.2 ) • Prevention of CMV disease in kidney and heart transplant patients at high risk 1.1 Adult Patients Treatment of Cytomegalovirus (CMV) Retinitis: Valganciclovir tablets are indicated for the treatment of CMV retinitis in patients with acquired immunodeficiency syndrome (AIDS) [see Clinical Studies ( 14.1 )]. Prevention of CMV Disease: Valganciclovir tablets are indicated for the prevention of CMV disease in kidney, heart, and kidney-pancreas transplant patients at high risk (Donor CMV seropositive/Recipient CMV seronegative [D+/R-]) [see Clinical Studies ( 14.1 )]. 1.2 Pediatric Patients Prevention of CMV Disease: Valganciclovir tablets are indicated for the prevention of CMV disease in kidney transplant patients (4 months to 16 years of age) and heart transplant patients (1 month to 16 years of age) at high risk [see Clinical Studies ( 14.2 )].
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Adult Dosage (2.2) Treatment of CMV retinitis Induction: 900 mg (two 450 mg tablets) twice a day for 21 days Maintenance: 900 mg (two 450 mg tablets) once a day Prevention of CMV disease in heart or kidney-pancreas transplant patients 900 mg (two 450 mg tablets) once a day within 10 days of transplantation until 100 days post-transplantation Prevention of CMV disease in kidney transplant patients 900 mg (two 450 mg tablets) once a day within 10 days of transplantation until 200 days post-transplantation Pediatric Dosage (2.3) Prevention of CMV disease in kidney transplant patients 4 months to 16 years of age Dose once a day within 10 days of transplantation until 200 days post-transplantation according to dosage algorithm (note the calculation of creatinine clearance using a modified Schwartz formula in children) Prevention of CMV disease in heart transplant patients 1 month to 16 years of age Dose once a day within 10 days of transplantation until 100 days post-transplantation according to dosage algorithm (note the calculation of creatinine clearance using a modified Schwartz formula in children) • Valganciclovir tablets should be taken with food. ( 2.1 , 12.3 ) • Valganciclovir tablets should not be broken or crushed. ( 2.6 ) • Adult patients should use valganciclovir tablets, not valganciclovir for oral solution. ( 2.1 ) • Adults with renal impairment: Adjust dose based on creatinine clearance. For adult patients receiving hemodialysis a dose recommendation cannot be given. 2.1 General Dosing Information • Adult patients should use valganciclovir tablets, not valganciclovir for oral solution. • Valganciclovir tablets should be taken with food [see Clinical Pharmacology ( 12.3 )]. 2.2 Recommended Dosage in Adult Patients with Normal Renal Function For dosage recommendations in adult patients with renal impairment [see Dosage and Administration ( 2.5 )]. Treatment of CMV Retinitis: • Induction: The recommended dosage is 900 mg (two 450 mg tablets) taken orally twice a day for 21 days. • Maintenance: Following induction treatment, or in adult patients with inactive CMV retinitis, the recommended dosage is 900 mg (two 450 mg tablets) taken orally once a day. Prevention of CMV Disease: • For adult patients who have received a heart or kidney-pancreas transplant, the recommended dosage is 900 mg (two 450 mg tablets) taken orally once a day starting within 10 days of transplantation until 100 days post-transplantation. • For adult patients who have received a kidney transplant, the recommended dosage is 900 mg (two 450 mg tablets) taken orally once a day starting within 10 days of transplantation until 200 days post-transplantation. 2.3 Recommended Dosage in Pediatric Patients Prevention of CMV Disease in Pediatric Kidney Transplant Patients: For pediatric kidney transplant patients 4 months to 16 years of age, the recommended once daily mg dose (7 x BSA x CrCl) should start within 10 days of post-transplantation until 200 days post-transplantation. Prevention of CMV Disease in Pediatric Heart Transplant Patients: For pediatric heart transplant patients 1 month to 16 years of age, the recommended once daily mg dose (7x BSA x CrCl) should start within 10 days of transplantation until 100 days post-transplantation. The recommended once daily dosage of valganciclovir tablets is based on body surface area (BSA) and creatinine clearance (CrCl) derived from a modified Schwartz formula, and is calculated using the equation below: Pediatric Dose (mg) = 7 x BSA x CrCl (calculated using a modified Schwartz formula). If the calculated Schwartz creatinine clearance exceeds 150 mL/min/1.73m 2 , then a maximum value of 150 mL/min/1.73m 2 should be used in the equation. The k values used in the modified Schwartz formula are based on pediatric patient age, as shown in Table 1. Table 1 k Values According to Pediatric Patient Age* k value Pediatric Patient Age 0.33 Infants less than 1 year of age with low birth weight for gestational age 0.45 Infants less than 1 year of age with birth weight appropriate for gestational age 0.45 Children aged 1 to less than 2 years 0.55 Boys aged 2 to less than 13 years Girls aged 2 to less than 16 years 0.7 Boys aged 13 to 16 years * The k values provided are based on the Jaffe method of measuring serum creatinine, and may require correction when enzymatic methods are used 1 . Monitor serum creatinine levels regularly and consider changes in height and body weight and adapt the dose as appropriate during prophylaxis period. All calculated doses should be rounded to the nearest 25 mg increment for the actual deliverable dose. The oral dispenser is graduated in 0.5 mL increments. A 50 mg dose is equivalent to 1 mL. If the calculated dose exceeds 900 mg, a maximum dose of 900 mg should be administered. Valganciclovir for oral solution is the preferred formulation since it provides the ability to administer a dose calculated according to the formula above; however, valganciclovir tablets may be used if the calculated doses are within 10% of available tablet strength (450 mg). For example, if the calculated dose is between 405 mg and 495 mg, one 450 mg tablet may be taken. Before prescribing valganciclovir tablets, pediatric patients should be assessed for the ability to swallow tablets. Formula formula2 2.5 Dosage Recommendation for Adult Patients with Renal Impairment Serum creatinine levels or estimated creatinine clearance should be monitored regularly during treatment. Dosage recommendations for adult patients with reduced renal function are provided in Table 2 . For adult patients on hemodialysis (CrCl less than 10 mL/min), a dose recommendation for valganciclovir tablets cannot be given [see Use in Specific Populations ( 8.5 , 8.6 ), Clinical Pharmacology ( 12.3 )]. Table 2 Dosage Recommendations for Adult Patients with Impaired Renal Function Valganciclovir Tablets 450 mg CrCl* (mL/min) Induction Dose Maintenance/Prevention Dose ≥ 60 900 mg twice daily 900 mg once daily 40 – 59 450 mg twice daily 450 mg once daily 25 – 39 450 mg once daily 450 mg every 2 days 10 – 24 450 mg every 2 days 450 mg twice weekly < 10 (on hemodialysis) not recommended not recommended *An estimated creatinine clearance in adults is calculated from serum creatinine by the following formulas: For males = (140 – age [years]) x (body weight [kg]) (72) x (serum creatinine [mg/dL]) For females = 0.85 x male value Dosing in pediatric patients with renal impairment can be done using the recommended equations because CrCl is a component in the calculation [see Dosage and Administration ( 2.3 )]. 2.6 Handling and Disposal Caution should be exercised in the handling of valganciclovir tablets. Tablets should not be broken or crushed. Because valganciclovir is considered a potential teratogen and carcinogen in humans, caution should be observed in handling broken tablets [see Warnings and Precautions ( 5.4 , 5.5 )]. Avoid direct contact with broken or crushed tablets with skin or mucous membranes. If such contact occurs, wash thoroughly with soap and water, and rinse eyes thoroughly with plain water. Handle and dispose valganciclovir tablets according to guidelines for antineoplastic drugs because ganciclovir shares some of the properties of antitumor agents (i.e., carcinogenicity and mutagenicity) 2.
