Drug Catalog - Product Detail
URSODIOL, USP TB 500MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68462-0474-01 | GLENMARK PHARMACEUTICALS | 100 | 500MG | TABLET |
PACKAGE FILES



Generic Name
URSODIOL
Substance Name
URSODIOL
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA090801
Description
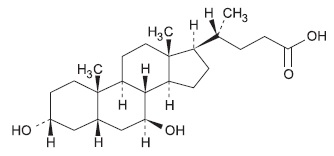
11 DESCRIPTION Ursodiol Tablets, USP 250 mg are available as film-coated tablets for oral administration. Ursodiol Tablets, USP 500 mg are available as scored film-coated tablets for oral administration. Ursodiol (ursodeoxycholic acid, UDCA) is a naturally occurring bile acid found in small quantities in normal human bile and in larger quantities in the biles of certain species of bears. It is a white to almost white crystalline powder, freely soluble in alcohol and glacial acetic acid, sparingly soluble in chloroform, slightly soluble in ether, and practically insoluble in water. The chemical name of ursodiol is 3α,7ß-dihydroxy-5ß-cholan-24-oic (C 24 H 40 O 4 ). Ursodiol has a molecular weight of 392.57. Its structure is shown below. Inactive ingredients: microcrystalline cellulose, povidone, sodium starch glycolate, magnesium stearate, ethylcellulose, dibutyl sebacate, carnauba wax, hypromellose, PEG 3350, PEG 8000, cetyl alcohol and sodium lauryl sulfate. structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 Ursodiol Tablets, USP 250 mg Each Ursodiol Tablet, USP 250 mg is a white to off-white, oval shaped, film-coated tablet debossed with “G72” on one side and “250” on the other side and contains 250 mg ursodiol, USP. Ursodiol Tablets, USP 250 mg are available in: Bottles of 30 tablets, NDC 68462-473-30 Bottles of 100 tablets, NDC 68462-473-01 Bottles of 500 tablets, NDC 68462-473-05 16.2 Ursodiol Tablets, USP 500 mg Each Ursodiol Tablet, USP 500 mg is a white to off-white, oval shaped, film-coated tablet debossed with “U 11” on one side and a score line on the other side and contains 500 mg ursodiol, USP. Ursodiol Tablets, USP 500 mg are available in: Bottles of 30 tablets, NDC 68462-474-30 Bottles of 100 tablets, NDC 68462-474-01 Bottles of 500 tablets, NDC 68462-474-05 Store at 20℃ to 25℃ (68℉ to 77℉) [see USP Controlled Room Temperature]. Dispense in a tight container. Half-tablets (scored Ursodiol Tablets, USP 500 mg broken in half) maintain acceptable quality for up to 28 days when stored in the current packaging (bottles) at 20℃ to 25℃ (68℉ to 77℉). Due to the bitter taste, the halved segments should be stored separately from the whole tablets [see Dosage and Administration ( 2.3 )].
Indications & Usage
1 INDICATIONS AND USAGE Ursodiol tablets, 250 mg and 500 mg are indicated for the treatment of patients with primary biliary cholangitis (PBC). Ursodiol tablets, 250 mg and 500 mg are bile acids indicated for the treatment of patients with primary biliary cholangitis. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION • Recommended adult dosage: 13 to 15 mg/kg/day administered in two to four divided doses with food. ( 2.1 ) • Scored ursodiol tablet, 500 mg: scored tablet can be broken in halves to provide recommended dosage. ( 2.3 , 16 ) 2.1 General Dosing Information The recommended adult dosage for ursodiol tablets, 250 mg and 500 mg in the treatment of PBC is 13 to 15 mg/kg/day administered in two to four divided doses with food. Dosing regimen should be adjusted according to each patient’s need at the discretion of the physician. 2.2 Liver Function Tests Liver function tests (γ-GT, alkaline phosphatase, AST, ALT) and bilirubin levels should be monitored every month for three months after start of therapy, and every six months thereafter [see Warnings and Precautions ( 5.1 )]. 2.3 Scoring the Ursodiol Tablet, 500 mg The ursodiol 500 mg scored tablet can be broken in halves to provide recommended dosage. To break the ursodiol 500 mg scored tablet easily, place the tablet on a flat surface with the scored section on top. Hold the tablet with your thumbs placed close to the scored part of the tablet (groove). Then apply gentle pressure and snap the tablet segments apart (segments breaking incorrectly should not be used). The segments should be washed down unchewed, with water, keeping the segments in the mouth can reveal a bitter taste. Due to the bitter taste, segments should be stored separately from whole tablets. [see How Supplied/Storage and Handling ( 16 )].
