Drug Catalog - Product Detail
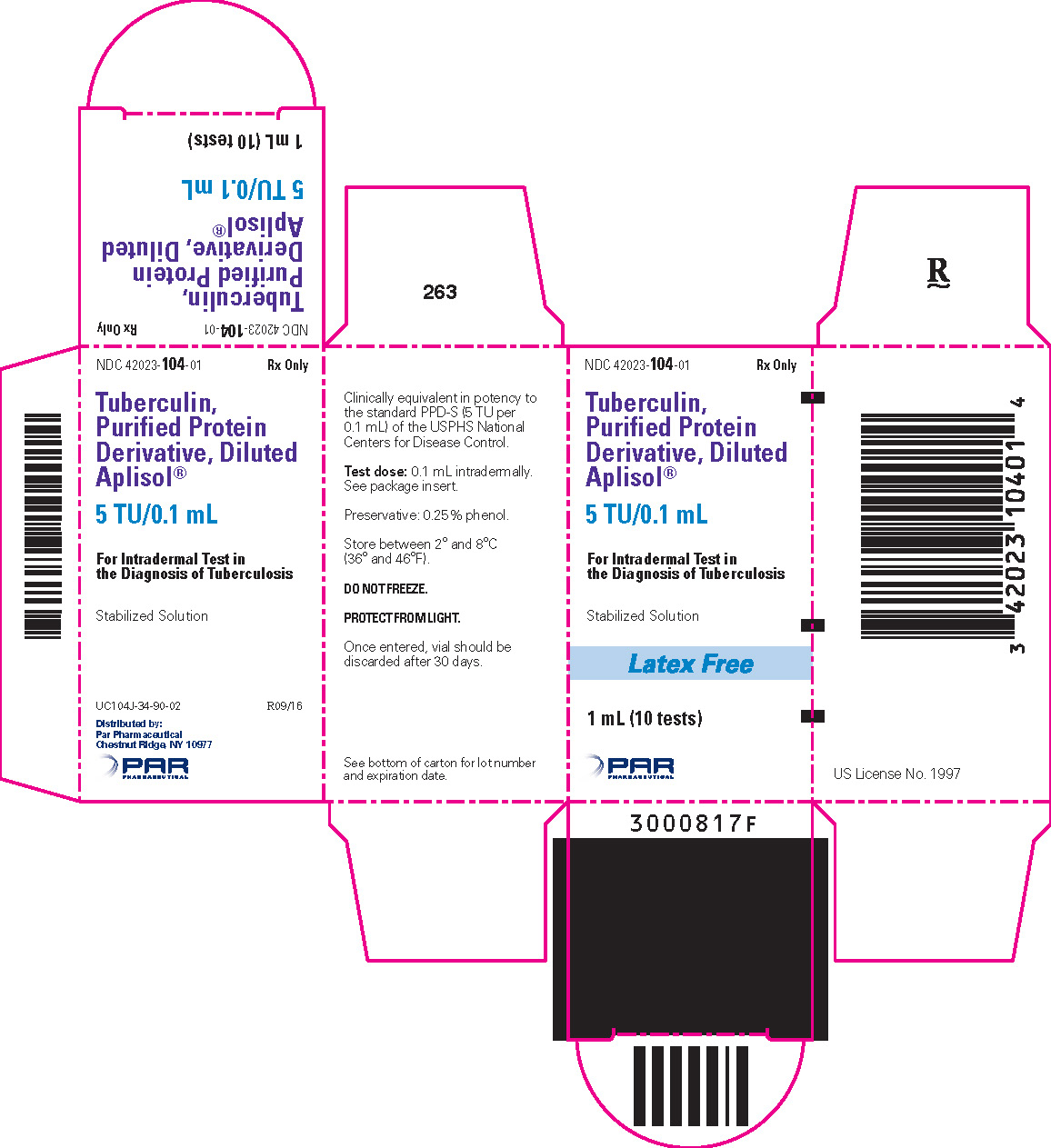
TUBERCULIN PURIFIED PROTEIN DERIVATIVE (APLISOL) INJECT. 5TU/0.1ML 1MLX1
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 42023-0104-01 | ENDO USA | 1 | 5UNIT/0.1ML | SOLUTION |
PACKAGE FILES
Generic Name
TUBERCULIN PURIFIED PROTEIN DERIVATIVE
Substance Name
TUBERCULIN PURIFIED PROTEIN DERIVATIVE
Product Type
HUMAN PRESCRIPTION DRUG
Route
INTRADERMAL
Application Number
BLA103782
Description
DESCRIPTION Aplisol (tuberculin PPD, diluted) is a sterile aqueous solution of a purified protein fraction for intradermal administration as an aid in the diagnosis of tuberculosis. The solution is stabilized with polysorbate (Tween) 80, buffered with potassium and sodium phosphates and contains approximately 0.25% phenol as a preservative. This product is ready for immediate use without further dilution. The purified protein fraction is isolated from culture media filtrates of a human strain of Mycobacterium tuberculosis by the method of F.B. Seibert. 1,2 Tuberculin PPD, diluted, is prepared from Tuberculin PPD which is clinically bioequivalent in potency to the standard PPD-S* (5 TU** per 0.1mL) of the U.S. Public Health Service, National Centers for Disease Control. The potency of each lot of tuberculin PPD, diluted is determined in sensitized guinea pigs.
How Supplied
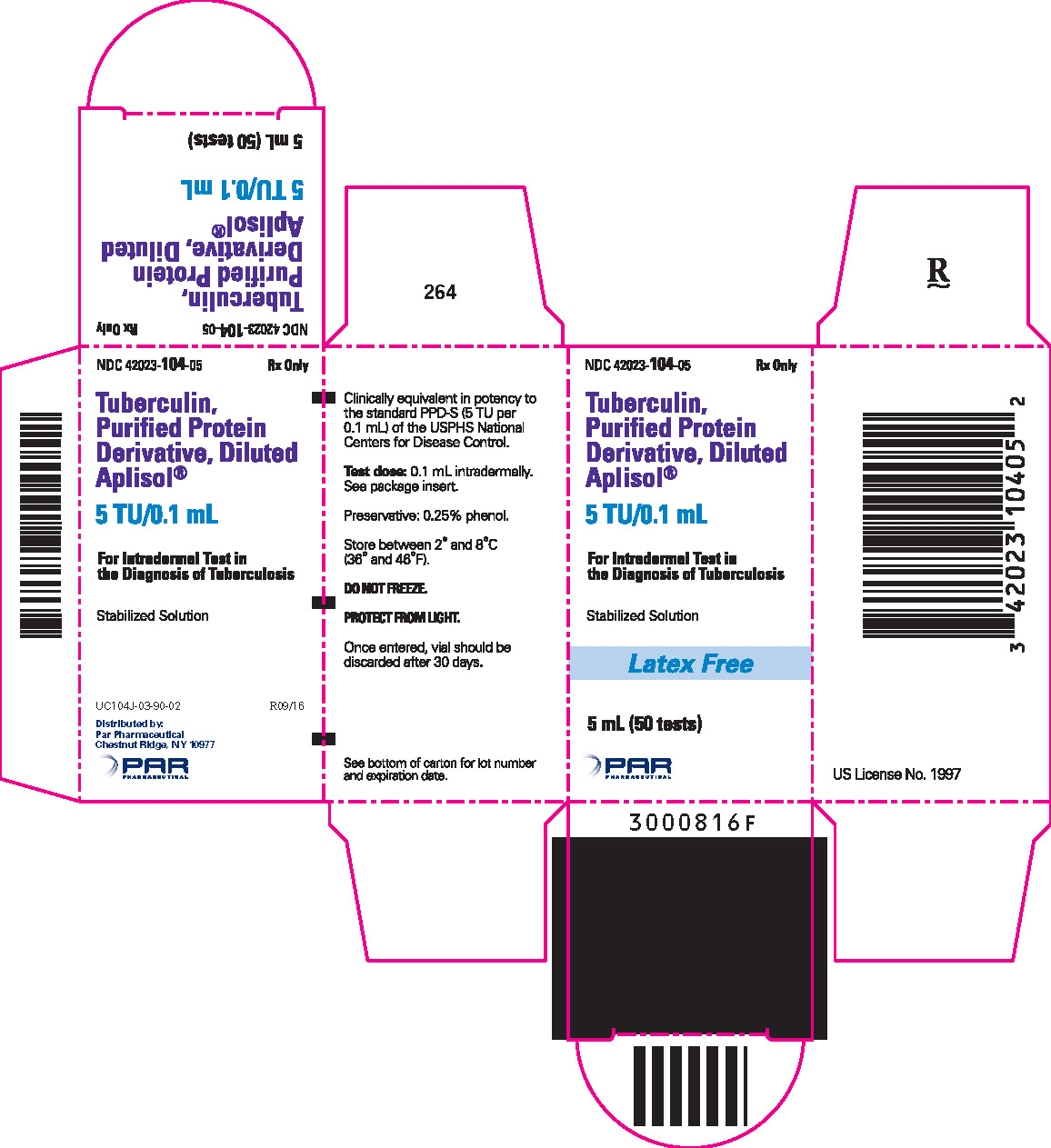
HOW SUPPLIED Tuberculin PPD-Aplisol bioequivalent to 5 US units (TU) PPD-S per test dose (0.1 mL) is available in the following presentations: NDC 42023-104-01 (Bio. 1525) 1 mL (10 tests) – multiple dose vial NDC 42023-104-05 (Bio.1607) 5 mL (50 tests) – multiple dose vial This product is ready for use without further dilution. Storage DO NOT FREEZE This product should be stored between 2° and 8°C (36° and 46°F) and protected from light. Vials in use more than 30 days should be discarded due to possible oxidation and degradation which may affect potency. *PPD-S - World Health Organization International PPD-Tuberculin Standard **U.S. Tuberculin Unit
Indications & Usage
INDICATIONS AND USAGE Tuberculin PPD is indicated as an aid in the detection of infection with Mycobacterium tuberculosis . The standard tuberculin test employs the intradermal (Mantoux) test using a 5 TU dose of tuberculin PPD. 7 The 0.1 mL test dose of Aplisol (tuberculin PPD, diluted) is equivalent to the 5 TU dose which has been clinically utilized and standardized with PPD-S. Tuberculin skin testing is not contraindicated for persons who have been vaccinated with BCG and the skin-test results of such persons are used to support or exclude the diagnosis of M. tuberculosis infections. 4 HIV infection is a strong risk factor for the development of TB disease in persons having TB infection. All HIV-infected persons should receive a PPD-tuberculin skin test. 3
Dosage and Administration
DOSAGE AND ADMINISTRATION Aplisol vials should be inspected visually for both particulate matter and discoloration prior to administration and discarded if either is seen. Vials in use for more than 30 days should be discarded. The 0.1 mL dose of Aplisol (tuberculin PPD, diluted) is equivalent to the 5 tuberculin units (TU) dose of Tuberculin PPD, which is the standard strength used for intradermal Mantoux testing. Standard Method (Mantoux Test) The Mantoux test is performed by intradermally injecting, on the volar aspect of the forearm, with a syringe and needle, exactly 0.1 mL of Aplisol. The result is read 48 to 72 hours later and palpable induration only is considered in interpreting the test . Induration is a hard, raised area with clearly defined margins at and around the injection site (see Interpretation of Tuberculin Reaction ). Erythema may develop at the injection site but has no diagnostic value. The standard test is performed as follows: The site of the test is usually the volar or dorsal surface of the forearm about 4" below the elbow. Other skin sites may be used, but the volar surface of the forearm is preferred. The use of a skin area free of lesions and away from any veins is recommended. 7 The skin at the injection site is cleansed with 70% alcohol and allowed to dry. The test material is administered with a tuberculin syringe (0.5 or 1.0 mL) fitted with a short (1/4 to 1/2") 27 gauge needle. A separate, sterile, single-use disposable syringe and needle should be used for each individual patient. The diaphragm of the vial-stopper should be wiped with 70% alcohol. The needle is inserted through the stopper diaphragm of the inverted vial. Exactly 0.1 mL is filled into the syringe with care being taken to exclude air bubbles and to maintain the lumen of the needle filled. The point of the needle is inserted into the most superficial layers of the skin with the needle bevel pointed upward. As the Tuberculin solution is injected, a pale bleb 6 to 10 mm in size (1/3") will rise over the point of the needle. This is quickly absorbed and no dressing is required. There may be a drop of blood when the needle is withdrawn. This is normal. Use a gauze pad and gently dab to remove the blood. Do not press down as this may squeeze out the tuberculin thereby disrupting the test. In the event the injection is delivered subcutaneously (i.e., no bleb will form), or if a significant part of the dose leaks from the injection site, the test should be repeated immediately at another site at least 5 cm (2") removed from the initial injection site. Interpretation of Tuberculin Reaction Readings of Mantoux reactions should be made by a trained health professional during the period from 48 to 72 hours after the injection. Induration only should be considered in interpreting the test. The diameter of induration should be measured transversely to the long axis of the forearm and recorded in millimeters. Erythema has no diagnostic value and should be disregarded. The presence and size of necrosis and edema if present should be recorded although not used in the interpretation of the test. In the absence of induration, an area of erythema greater than 10 mm in diameter may indicate the injection was made too deeply and retesting is indicated. Find the margins of the induration by drawing the index or middle finger lightly across the reaction. The tip of a ballpoint pen pushed at a 45° angle toward the site of injection will also stop at the edges of induration. The diameter of induration should be measured (preferable with a caliper) transversely to the long axis of the forearm and recorded in millimeters. Erythema has no diagnostic value and should be disregarded. The absence of induration should be recorded as "0 mm" not "negative". Reactions should be interpreted as follows (Please refer to most recent guidelines): Based on current guidelines, 3,7,14, 19 interpretation of reactions is as follows: Positive reactions: Reaction ≥ 5 mm of Induration Reaction ≥ 10 mm of Induration Reaction ≥ 15 mm of Induration Human immunodeficiency virus (HIV)-positive persons Recent immigrants (i.e., within the last 5 yr) from high prevalence countries Persons with no risk factors for TB Recent contacts of tuberculosis (TB) case patients Injection drug users Fibrotic changes on chest radiograph consistent with prior TB Residents and employees For persons who are otherwise at low risk and are tested at the start of employment, a reaction of ≥ 15 mm induration is considered positive. of the following high-risk congregate settings: prisons and jails, nursing homes and other long-term facilities for the elderly, hospitals and other health care facilities, residential facilities for patients with acquired immunodeficiency syndrome (AIDS), and homeless shelters Patients with organ transplants and other immunosuppressed patients (receiving the equivalent of ≥ 15 mg/d of prednisone for 1 mo or more) Risk of TB in patients treated with corticosteroids increases with higher dose and longer duration. 19 Mycobacteriology laboratory personnel Persons with the following clinical conditions that place them at high risk: silicosis, diabetes mellitus, chronic renal failure, some hematological disorders (e.g., leukemias and lymphomas), other specific malignancies (e.g., carcinoma of the head or neck and lung), weight loss of ≥ 10% of ideal body weight, gastrectomy, and jejunoileal bypass Children younger than 4 yr of age or infants, children, and adolescents exposed to adults at high-risk Skin test conversions For persons with negative skin test reactions who undergo repeat skin testing (e.g., health care workers), an increase in reaction size ≥ 10 mm within a period of 2 years should be considered a skin test conversion indicative of recent infection with M. tuberculosis . 19 In some individuals who have been infected with nontuberculous mycobacteria or have undergone BCG vaccination, the skin test may show some degree of induration. For these individuals, a conversion to "positive" is defined as an increase in induration by 10 mm on subsequent tests. 7 Healthcare facilities and other high-risk settings For health care workers and employees in other high-risk settings with no other risk factors for TB, a cut-off of 15 mm of induration (rather than 10 mm) on the tuberculin skin test should be used to define a positive baseline test at the time of initial employment. An increase of ≥10 mm in reaction size is generally accepted as a positive test result on subsequent testing unless the worker is a contact of a TB case or has HIV infection or is otherwise immunocompromised, in which case a result of ≥5 mm is considered positive. 21 Negative Reaction A negative reaction is an induration of less than 15 mm in persons with no risk factors for TB. This indicates a lack of hypersensitivity to tuberculoprotein and tuberculous infection is highly unlikely. 7 It should be noted that reactivity to tuberculin may be depressed or suppressed for as long as 5–6 weeks by viral infections, live virus vaccines (i.e., measles, smallpox, polio, rubella and mumps), or after discontinuation of therapy with corticosteroids or immunosuppressive agents. Malnutrition may also have a similar effect (see WARNINGS ). When of diagnostic importance, a negative test should be accepted as proof that hypersensitivity is absent only after normal reactivity to non-specific irritants has been demonstrated. A primary injection of tuberculin may possibly have a boosting effect on subsequent tuberculin reactions. A pediatric patient who is known to have been exposed to a person with tuberculosis must not be adjudged free of infection until that patient has a negative tuberculin reaction at least ten weeks after contact with tuberculous person has ceased. 17 Annual testing is generally recommended for pediatric patients in high risk populations, such as persons from countries with a high prevalence of tuberculosis and low-income groups. 18 A positive tuberculin reaction does not necessarily signify the presence of active disease. Further diagnostic procedures (e.g., chest radiograph, sputum smear and/or culture examination) should be carried out before a diagnosis of tuberculosis is made. A small percentage of responders may not have been infected with M. tuberculosis but by some other mycobacterium. The negative tuberculin skin test should never be used to exclude the possibility of active tuberculosis among persons for whom the diagnosis is being considered (symptoms compatible with tuberculosis). Booster Effect and Two-Step Testing Infection of an individual with tubercle bacilli or other mycobacteria or BCG vaccination results in a delayed hypersensitivity response to tuberculin which is demonstrated by the skin test. The delayed hypersensitivity response may gradually wane over a period of years. If a person receives a tuberculin test at this time, a significant reaction may not be detected. However, the stimulus of the test may boost or increase the size of the reaction to a second test, sometimes causing an apparent conversion or development of sensitivity. This booster effect can be seen on a second test done one week after the initial stimulating test and can persist for a year, and perhaps longer. When routine periodic tuberculin testing of adults is done, initially two-stage testing should be considered to minimize the likelihood of interpreting a boosted reaction as a conversion. 7,15,16 In this testing method, persons who have a negative initial skin test undergo a second tuberculin skin test 1-3 weeks after the first. Both tests should be read and recorded at 48 to 72 hours. Those individuals with a positive reaction on the second test should be considered to be previously infected, and those with a negative reaction on the second test should be considered uninfected. In these uninfected persons, a positive result on any future skin test should be interpreted as a skin test conversion. 7
