Drug Catalog - Product Detail
TRIAMCINOLONE ACET DENTAL USP PSTE 0.001 5GM
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 64980-0320-05 | RISING PHARMACEUTICALS | 5 | 0.1% | PASTE |
PACKAGE FILES
Generic Name
TRIAMCINOLONE ACETONIDE
Substance Name
TRIAMCINOLONE ACETONIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
ANDA040771
Description
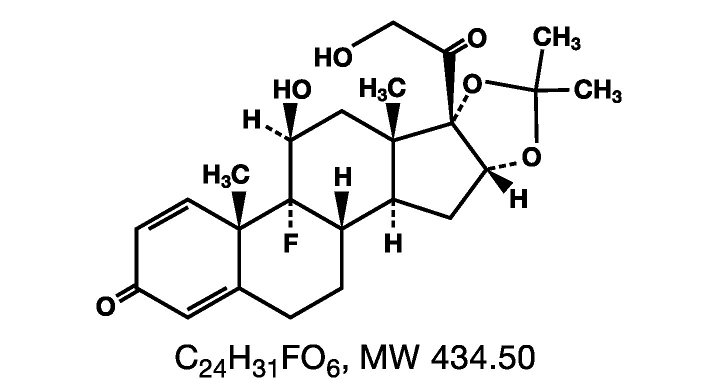
DESCRIPTION Triamcinolone Acetonide Dental Paste USP, 0.1% contains the corticosteroid triamcinolone acetonide in an adhesive vehicle suitable for application to oral tissues. Triamcinolone acetonide is designated chemically as 9-fluoro-11β, 16α, 17, 21-tetrahydroxypregna-1, 4-diene-3, 20-dione cyclic 16,17-acetal with acetone. The structural formula of triamcinolone acetonide is as follows: Each gram of Triamcinolone Acetonide Dental Paste USP, 0.1% contains 1 mg triamcinolone acetonide in a dental paste containing gelatin, pectin, cream flavor, vanilla flavor and carboxymethylcellulose sodium in Plasticized Hydrocarbon Gel, a polyethylene and mineral oil gel base. LABEL
How Supplied
HOW SUPPLIED Triamcinolone Acetonide Dental Paste USP, 0.1% Tubes containing 5 g of dental paste NDC 64980-320-05
Indications & Usage
INDICATIONS AND USAGE Triamcinolone Acetonide Dental Paste USP, 0.1% is indicated for adjunctive treatment and for the temporary relief of symptoms associated with oral inflammatory lesions and ulcerative lesions resulting from trauma.
Dosage and Administration
DOSAGE AND ADMINISTRATION Press a small dab (about 1/4 inch) to the lesion until a thin film develops. A larger quantity may be required for coverage of some lesions. For optimal results use only enough to coat the lesion with a thin film. Do not rub in. Attempting to spread this preparation may result in granular, gritty sensation and cause it to crumble. After application, however, a smooth, slippery film develops. The preparation should be applied at bedtime to permit steroid contact with the lesion throughout the night. Depending on the severity of symptoms, it may be necessary to apply the preparation two or three times a day, preferably after meals. If significant repair or regeneration has not occurred in seven days, further investigation is advisable.
