Drug Catalog - Product Detail
TRETINOIN CREAM 0.1% 45GM
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00574-2201-45 | PADAGIS | 45 | 0.1% | CREAM |
PACKAGE FILES








Generic Name
TRETINOIN
Substance Name
TRETINOIN
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
ANDA075213
Description
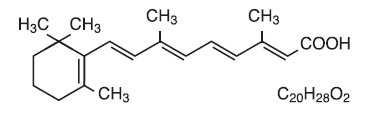
Description: Tretinoin Gel, USP and Tretinoin Cream, USP are used for the topical treatment of acne vulgaris. Each gram of tretinoin gel contains tretinoin in either of two strengths, 0.025% (0.25 mg) or 0.01% (0.1 mg) in a gel vehicle of hydroxypropyl cellulose, butylated hydroxytoluene, and alcohol 90% w/w. Each gram of tretinoin cream contains tretinoin in either of three strengths, 0.1% (1 mg), 0.05% (0.5 mg), or 0.025% (0.25 mg) in a hydrophilic cream vehicle of: stearic acid, isopropyl myristate, polyoxyl 40 stearate, stearyl alcohol, xanthan gum, sorbic acid, butylated hydroxytoluene, and purified water. Chemically, tretinoin is all-trans -retinoic acid. It has a molecular weight of 300.44 and has the following structural formula: chemical-structure-tretinoin.jpg
How Supplied
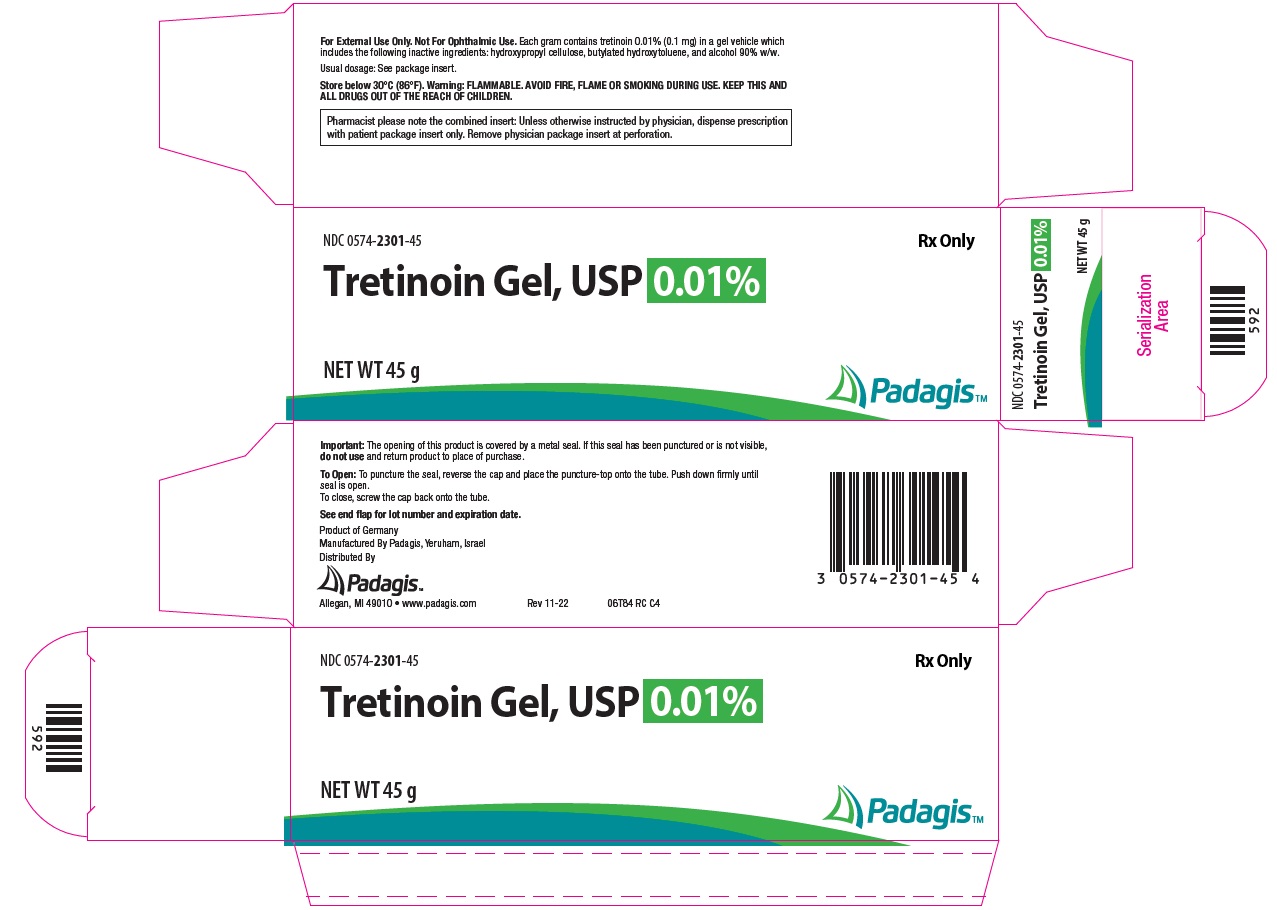
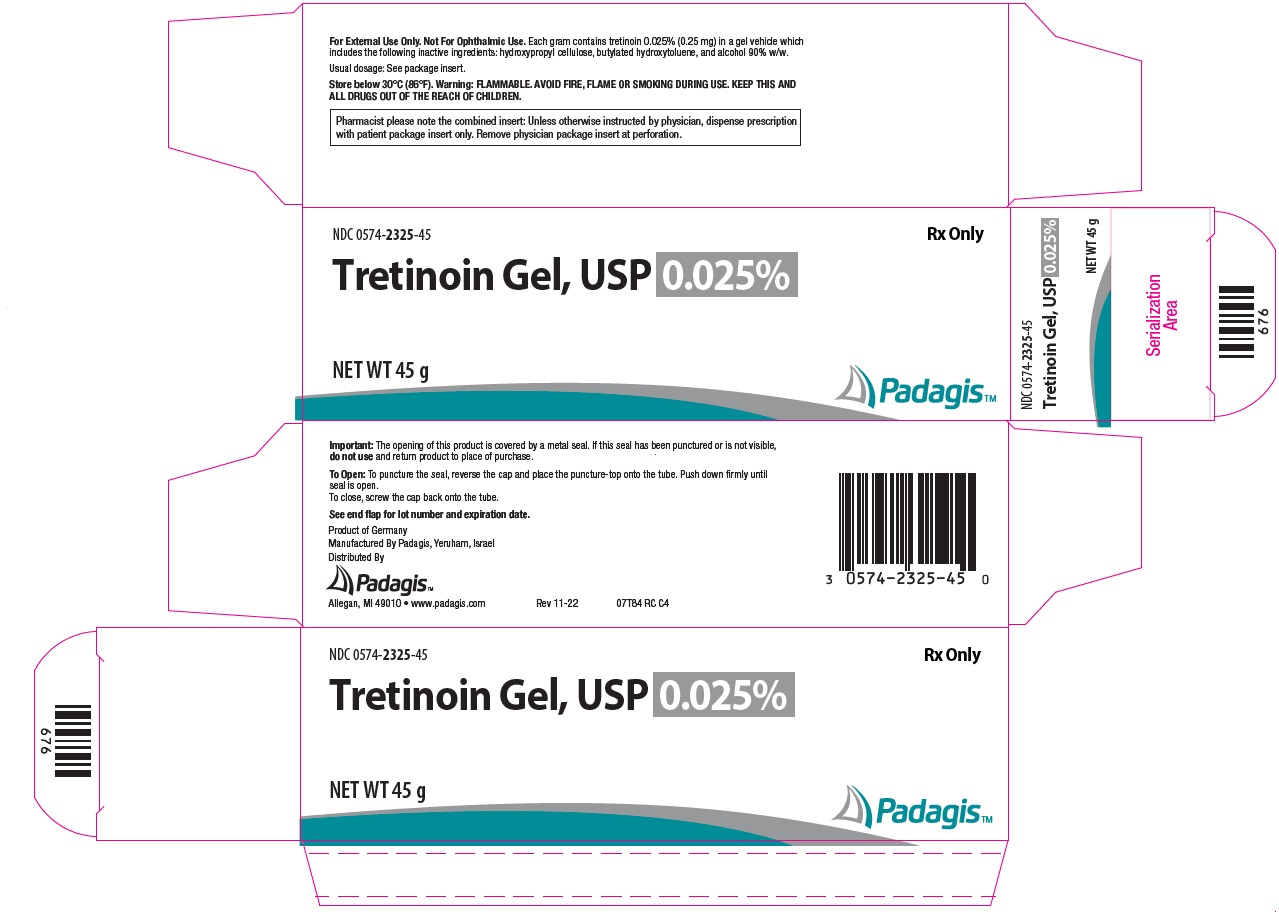
How Supplied: Tretinoin Gel, USP NDC CODE Strength Qty. 0574-2325-15 0.025% 15 g 0574-2325-45 0.025% 45 g 0574-2301-15 0.01% 15 g 0574-2301-45 0.01% 45 g Tretinoin Cream, USP NDC CODE Strength Qty. 0574-2201-20 0.1% 20 g 0574-2201-45 0.1% 45 g 0574-2205-20 0.05% 20 g 0574-2205-45 0.05% 45 g 0574-2225-20 0.025% 20 g 0574-2225-45 0.025% 45 g
Indications & Usage
Indications and Usage: Tretinoin gel and cream are indicated for topical application in the treatment of acne vulgaris. The safety and efficacy of the long-term use of this product in the treatment of other disorders have not been established.
Dosage and Administration
Dosage and Administration: Tretinoin gel or cream should be applied once a day, before retiring, to the skin where acne lesions appear, using enough to cover the entire affected area lightly. Gel: Excessive application results in “pilling” of the gel, which minimizes the likelihood of over application by the patient. Application may cause a transitory feeling of warmth or slight stinging. In cases where it has been necessary to temporarily discontinue therapy or to reduce the frequency of application, therapy may be resumed or frequency of application increased when the patients become able to tolerate the treatment. Alterations of vehicle, drug concentration, or dose frequency should be closely monitored by careful observation of the clinical therapeutic response and skin tolerance. During the early weeks of therapy, an apparent exacerbation of inflammatory lesions may occur. This is due to the action of the medication on deep, previously unseen lesions and should not be considered a reason to discontinue therapy. Therapeutic results should be noticed after two to three weeks but more than six weeks of therapy may be required before definite beneficial effects are seen. Once the acne lesions have responded satisfactorily, it may be possible to maintain the improvement with less frequent applications, or other dosage forms. Patients treated with tretinoin preparations may use cosmetics, but the areas to be treated should be cleansed thoroughly before the medication is applied ( see Precautions ).
