Drug Catalog - Product Detail
TRANYLCYPROMINE SULFATE TB 10MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00591-5590-01 | ACTAVIS PHARMA | 100 | 10MG | TABLET |
PACKAGE FILES
Generic Name
TRANYLCYPROMINE SULFATE
Substance Name
TRANYLCYPROMINE SULFATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
NDA012342
Description
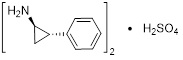
11 DESCRIPTION Tranylcypromine sulfate, the active ingredient of tranylcypromine sulfate tablets, is a non-hydrazine MAOI. The chemical name is (±)‑ trans ‑2‑phenylcyclopropylamine sulfate (2:1). The molecular formula is (C 9 H 11 N) 2 •H 2 SO 4 and its molecular weight is 364.46. The structural formula is: Tranylcypromine sulfate tablets film-coated tablets are intended for oral administration. Each round, rose‑red tablet is debossed on one side with the product name “PARNATE” and “SB” and contains tranylcypromine sulfate equivalent to 10 mg of tranylcypromine. Inactive ingredients consist of microcrystalline cellulose, anhydrous citric acid, croscarmellose sodium, D&C Red No. 7, FD&C Blue No. 2, FD&C Yellow No. 6, gelatin, lactose, magnesium stearate, talc, titanium dioxide, carnauba wax, polyethylene glycol 400 and 8000, and hypromellose. structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Tranylcypromine sulfate tablets are available as: 10 mg: film-coated, round, rose-red and debossed with the product name “PARNATE” and “SB” on one side of the tablet containing tranylcypromine sulfate equivalent to 10 mg of tranylcypromine. Bottles of 100 tablets: NDC 0591-5590-01 Store between 15° and 30°C (59° and 86°F). Dispense in a tight, light resistant container.
Indications & Usage
1 INDICATIONS & USAGE Tranylcypromine sulfate tablets are indicated for the treatment of major depressive disorder (MDD) in adult patients who have not responded adequately to other antidepressants. Tranylcypromine sulfate tablets are not indicated for the initial treatment of MDD due to the potential for serious adverse reactions and drug interactions, and the need for dietary restrictions [see Contraindications (4), Warnings and Precautions (5) , and Drug Interactions (7)]. Tranylcypromine sulfate tablets are a monoamine oxidase inhibitor (MAOI) indicated for the treatment of major depressive disorder (MDD) in adult patients who have not responded adequately to other antidepressants ( 1 ) Tranylcypromine sulfate tablets are not indicated for the initial treatment of MDD due to the potential for serious adverse reactions and drug interactions, and the need for dietary restrictions ( 1 , 4 , 5 , 7 )
Dosage and Administration
2 DOSAGE & ADMINISTRATION Recommended daily dosage is 30 mg in divided doses (2.1) If no adequate response, increase dosage in increments of 10 mg per day every 1 to 3 weeks to a maximum dosage of 30 mg twice daily (60 mg per day).Consider more gradual dosage increases in patients at risk for hypotension (2.1) Consider discontinuing tranylcypromine sulfate tablets therapy gradually because of the risk for withdrawal effects ( 2.3 , 5.8 , 9.3 ) Switching from or to other MAOIs or other antidepressants: See full prescribing information for instructions ( 2.2 , 7.1 ) 2.1 Recommended Dosage Tranylcypromine sulfate tablets are for oral use. The recommended dosage is 30 mg per day (in divided doses). If patients do not have an adequate response, increase the dosage in increments of 10 mg per day every 1 to 3 weeks to a maximum 30 mg twice daily (60 mg per day). Dosage increases should be made more gradually in patients at risk for hypotension (e.g., geriatric patients) [see Warnings and Precautions (5.5)]. 2.2 Switching to or from Other Antidepressants Switching from Contraindicated Antidepressants to Tranylcypromine Sulfate Tablets After stopping treatment with contraindicated antidepressants, a time period of 4 to 5 half-lives of the other antidepressant or any active metabolite should elapse before starting treatment with tranylcypromine sulfate tablets. After stopping treatment with an MAO inhibitor antidepressant, a time period of at least one week or 4 to 5 half-lives of the other MAO inhibitor (whichever is longer) should elapse before starting treatment with tranylcypromine sulfate tablets to reduce the risk of additive effects [see Contraindications (4.1) and Drug Interactions (7.1)]. Switching from Tranylcypromine Sulfate Tablets to Other MAOIs or Contraindicated Antidepressants After stopping tranylcypromine sulfate tablets treatment, at least one week should elapse before starting another MAOI (intended to treat MDD) or other contraindicated antidepressants.Refer to the prescribing information of the subsequently used drug for product-specific advice on a medication-free interval [ see Contraindications (4.1) and Drug Interactions (7.1)]. 2.3 Discontinuing Treatment Withdrawal effects, including delirium, have been reported with abrupt discontinuation of tranylcypromine sulfate tablets therapy. Higher daily doses and longer duration of use appear to be associated with a higher risk of withdrawal effects. Consider discontinuing tranylcypromine sulfate tablets therapy by slow, gradual dosage reduction [see Warnings and Precautions (5.8) and Drug Abuse and Dependence (9.3)]. 2.4 Screen for Bipolar Disorder and Elevated Blood Pressure Prior to Starting Tranylcypromine Sulfate Tablets Prior to initiating treatment with tranylcypromine sulfate tablets: Screen patients for a history of mania [see Warnings and Precautions(5.4)]. Measure blood pressure [see Warnings and Precautions (5.2, 5.5)].
