Drug Catalog - Product Detail
TOBRAMYCIN SULF INHALATION SOLUTION 300MG/5ML 5MLx56
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65162-0914-46 | AMNEAL PHARMACEUTICALS | 5 | 300MG/5ML | NEBULIZER SOLUTION |
PACKAGE FILES



Generic Name
TOBRAMYCIN
Substance Name
TOBRAMYCIN
Product Type
HUMAN PRESCRIPTION DRUG
Route
RESPIRATORY (INHALATION)
Application Number
ANDA205501
Description
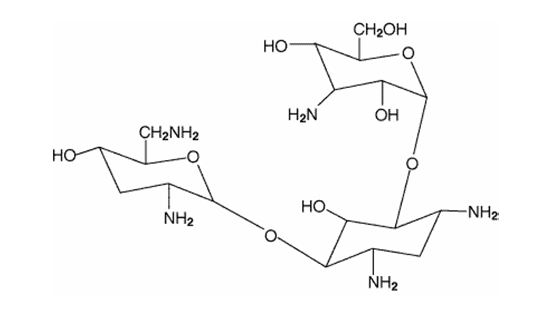
11 DESCRIPTION Tobramycin inhalation solution, USP is a tobramycin solution for inhalation. It is a sterile, clear, slightly yellow, non-pyrogenic, aqueous solution with the pH and salinity adjusted specifically for administration by a compressed air driven reusable nebulizer. The chemical formula for tobramycin is C 18 H 37 N 5 O 9 and the molecular weight is 467.52. Tobramycin is O-3-amino-3-deoxy-α- D-glucopyranosyl-(1→4)-O-[2,6-diamino-2,3,6- trideoxy-α-D- ribo -hexopyranosyl-(1→6)]-2-deoxy-L-streptamine. The structural formula for tobramycin is: Each single-dose 5 mL ampule contains 300 mg tobramycin, USP and 11.25 mg sodium chloride in sterile water for injection. Sulfuric acid and sodium hydroxide are added to adjust the pH to 6.0. Nitrogen is used for sparging. All ingredients meet USP requirements. The formulation contains no preservatives. 10
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied Tobramycin inhalation solution USP is supplied as a sterile, clear, slightly yellow, non-pyrogenic, aqueous solution packaged in a 5 mL single-dose ampule (300 mg tobramycin) for nebulization. It is available as follows: 5 mL single-dose ampule packaged in cartons containing 56 ampules (14 pouches, each containing 4 ampules) NDC 65162-914-46 16.2 Storage and Handling Tobramycin inhalation solution should be stored under refrigeration at 2º to 8ºC or 36º to 46ºF. Upon removal from the refrigerator, or if refrigeration is unavailable, tobramycin inhalation solution pouches (opened or unopened) may be stored at room temperature (up to 25ºC/77ºF) for up to 28 days. Tobramycin inhalation solution should not be used beyond the expiration date stamped on the ampule when stored under refrigeration (2º to 8ºC or 36º to 46ºF) or beyond 28 days when stored at room temperature (25ºC/77ºF). Tobramycin inhalation solution ampules should not be exposed to intense light. The solution in the ampule is slightly yellow, but may darken with age if not stored in the refrigerator; however, the color change does not indicate any change in the quality of the product as long as it is stored within the recommended storage conditions.
Indications & Usage
1 INDICATIONS AND USAGE Tobramycin inhalation solution is indicated for the management of cystic fibrosis in adults and pediatric patients 6 years of age and older with Pseudomonas aeruginosa . Safety and efficacy have not been demonstrated in patients under the age of 6 years, patients with forced expiratory volume in 1 second (FEV 1 ) < 25% or > 75% predicted, or patients colonized with Burkholderia cepacia [see Clinical Studies (14) ]. Tobramycin is an aminoglycoside antibacterial indicated for the management of cystic fibrosis in adults and pediatric patients 6 years of age and older with Pseudomonas aeruginosa . ( 1 ) Safety and efficacy have not been demonstrated in patients under the age of 6 years, patients with forced expiratory volume in 1 second (FEV 1 ) < 25% or > 75% predicted, or patients colonized with Burkholderia cepacia. ( 1 )
Dosage and Administration
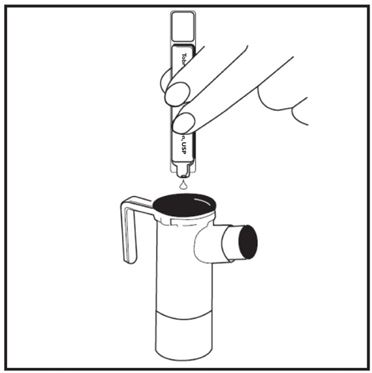
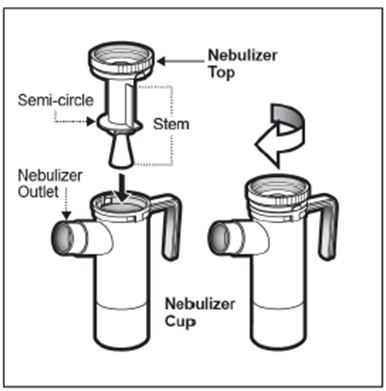
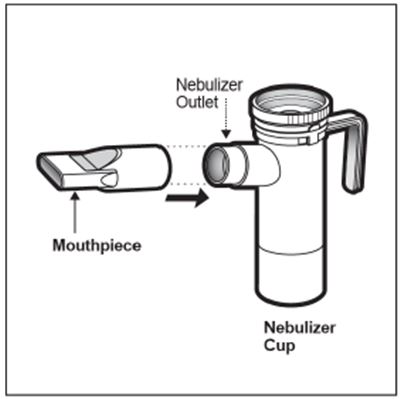
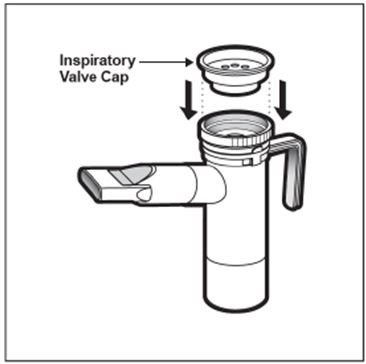
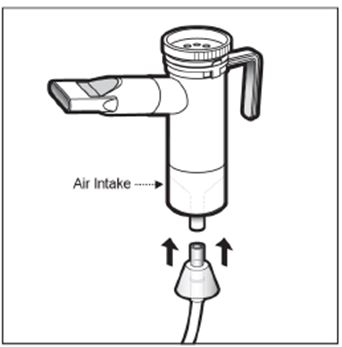
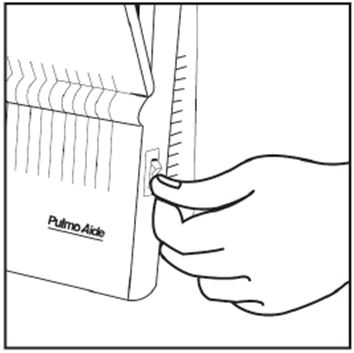
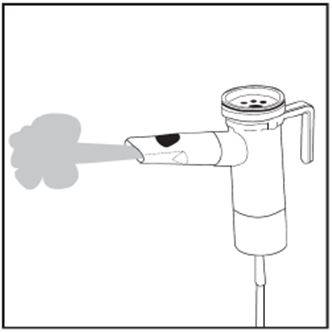
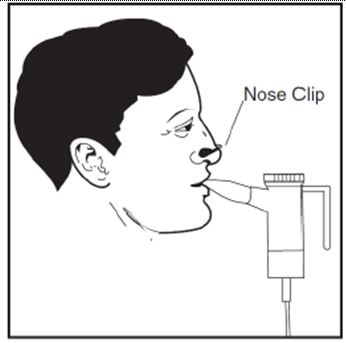
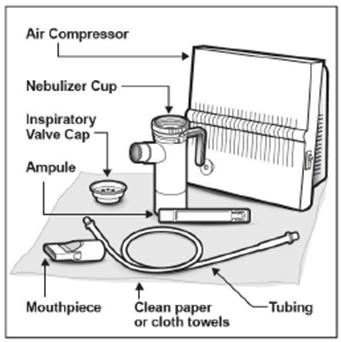
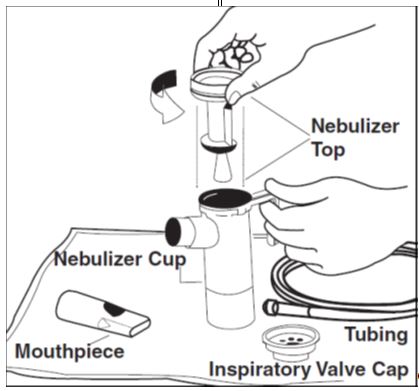
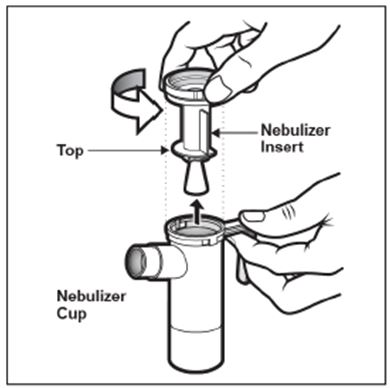
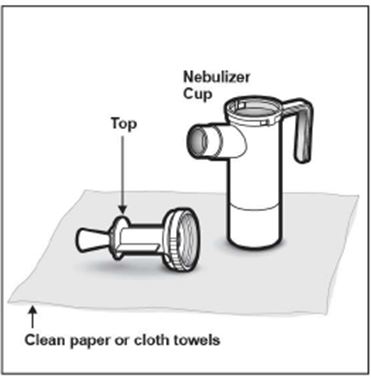
2 DOSAGE AND ADMINISTRATION For oral inhalation only. ( 2.1 ) The recommended dosage for adults and pediatric patients 6 years of age and older is one single-dose ampule (300 mg) twice daily by oral inhalation in alternating periods of 28 days on drug, followed by 28 days off drug. ( 2.1 ) Dosage is not adjusted by weight. ( 2.1 ) Take doses as close to 12 hours apart as possible; but not less than 6 hours apart. ( 2.1 ) Administer each 300 mg dose by inhalation using a hand-held PARI LC PLUS Reusable Nebulizer with a DeVilbiss Pulmo-Aide compressor. ( 2.2 ) 2.1 Dosage Tobramycin inhalation solution is for oral inhalation only [see Dosage and Administration (2.2) ] . The recommended dosage of tobramycin inhalation solution for both adults and pediatric patients 6 years of age and older is one single-dose ampule (300 mg) administered twice daily for 28 days. Dosage is not adjusted by weight. All patients should be administered 300 mg twice daily. Tobramycin inhalation solution is administered twice daily in alternating periods of 28 days. After 28 days of therapy, patients should stop tobramycin inhalation solution therapy for the next 28 days, and then resume therapy for the next 28 day on/28 day off cycle. The doses should be taken as close to 12 hours apart as possible; they should not be taken less than 6 hours apart. If patients miss a dose, they should take it as soon as possible anytime up to 6 hours prior to their next scheduled dose. If less than 6 hours remain before the next dose, wait until their next scheduled dose. 2.2 Administration Instructions Tobramycin inhalation solution is administered by oral inhalation over an approximately 15-minute period, using a hand-held PARI LC PLUS Reusable Nebulizer with a DeVilbiss Pulmo-Aide compressor. Tobramycin inhalation solution should not be diluted or mixed with dornase alfa or other medications in the nebulizer. Tobramycin inhalation solution is not for subcutaneous, intravenous or intrathecal administration. Prior to administration of tobramycin inhalation solution, read the Patient Information/Instructions for Use for tobramycin inhalation solution for detailed information on how to use tobramycin inhalation solution, and follow the manufacturer’s instructions for use and care of the PARI LC PLUS Reusable Nebulizer and DeVilbiss Pulmo-Aide air compressor. Tobramycin inhalation solution is inhaled while the patient is sitting or standing upright and breathing normally through the mouthpiece of the nebulizer. Nose clips may help the patient breathe through the mouth. Instruct patients on multiple therapies to take their medications, prior to inhaling tobramycin inhalation solution or as directed by their physician. Tobramycin inhalation solution should not be used if it is cloudy, if there are particles in the solution, or if it has been stored at room temperature for more than 28 days.
