Drug Catalog - Product Detail
TIZANIDINE HCL TABS. TB 4MG 150
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 55111-0180-15 | DR.REDDY'S LABORATORIES, INC. | 150 | 4MG | TABLET |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
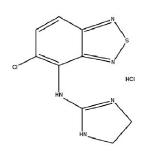
DESCRIPTION Tizanidine hydrochloride USP, is a centrally acting α 2 -adrenergic agonist. Tizanidine HCl USP (tizanidine) is a white to off-white, fine crystalline powder, which is odorless or with a faint characteristic odor. Tizanidine is slightly soluble in water and methanol; solubility in water decreases as the pH increases. Its chemical name is 5-chloro-4-(2-imidazolin-2-ylamino)-2,1,3-benzothiodiazole hydrochloride. Tizanidine’s molecular formula is C 9 H 8 ClN 5 S.HCl, its molecular weight is 290.2 and its structural formula is: Tizanidine tablets USP, is supplied as 2 mg and 4 mg tablets for oral administration. Tizanidine tablets USP, are composed of the active ingredient, tizanidine hydrochloride USP (2.29 mg equivalent to 2 mg tizanidine base and 4.58 mg equivalent to 4 mg tizanidine base), and the inactive ingredients, anhydrous lactose, microcrystalline cellulose, colloidal silicon dioxide and stearic acid. Structure
How Supplied
HOW SUPPLIED Tizanidine Tablets USP, 2 mg are white to off white, oval, flat, beveled edged tablets embossed with “R179” on one side and “bisecting score” on other side. The tablets are available in bottles of 30, 150, 300 and 1000. Bottles of 30 NDC 55111-179-30 Bottles of 150 NDC 55111-179-15 Bottles of 300 NDC 55111-179-03 Bottles of 1000 NDC 55111-179-10 Tizanidine Tablets USP, 4 mg are white to off white, oval, flat, beveled edged tablets embossed with “R180” on one side and “quadrisecting score” on other side. The tablets are available in bottles of 30, 150, 300 and 1000. Bottles of 30 NDC 55111-180-30 Bottles of 150 NDC 55111-180-15 Bottles of 300 NDC 55111-180-03 Bottles of 1000 NDC 55111-180-10 Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Dispense in containers with child resistant closure. Rx Only Manufactured by: Dr. Reddy’s Laboratories Limited Bachepalli – 502 325 INDIA Revised: 0909
Indications & Usage
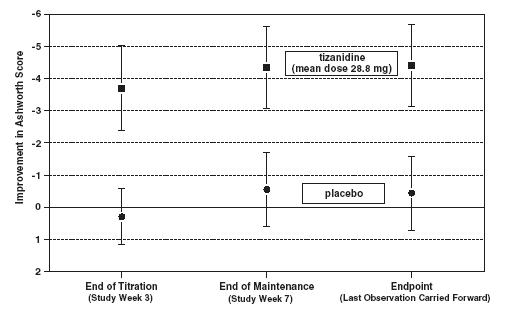
INDICATIONS AND USAGE Tizanidine tablets are a short-acting drug for the management of spasticity. Because of the short duration of effect, treatment with tizanidine should be reserved for those daily activities and times when relief of spasticity is most important (see DOSAGE AND ADMINISTRATION ).
Dosage and Administration
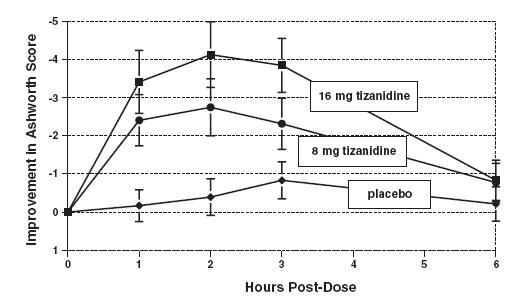
DOSAGE AND ADMINISTRATION A single dose of 8 mg of tizanidine reduces muscle tone in patients with spasticity for a period of several hours. The effect peaks at approximately 1 to 2 hours and dissipates between 3 to 6 hours. Effects are dose-related. Although single doses of less than 8 mg have not been demonstrated to be effective in controlled clinical studies, the dose-related nature of tizanidine’s common adverse events make it prudent to begin treatment with single oral doses of 4 mg. Increase the dose gradually (2 to 4 mg steps) to optimum effect (satisfactory reduction of muscle tone at a tolerated dose). The dose can be repeated at 6 to 8 hour intervals, as needed, to a maximum of three doses in 24 hours. The total daily dose should not exceed 36 mg. Experience with single doses exceeding 8 mg and daily doses exceeding 24 mg is limited. There is essentially no experience with repeated, single, daytime doses greater than 12 mg or total daily doses greater than 36 mg (see WARNINGS ). Food has complex effects on tizanidine pharmacokinetics, which differ with the different formulations. These pharmacokinetic differences may result in clinically significant differences when switching administration of the tablet between the fed or fasted state. These changes may result in increased adverse events or delayed/more rapid onset of activity, depending upon the nature of the switch. For this reason, the prescriber should be thoroughly familiar with the changes in kinetics associated with these different conditions (see CLINICAL PHARMACOLOGY: Pharmacokinetics ).
