Drug Catalog - Product Detail
THEOPHYLLINE ER ORAL TABLET EXTENDED RELEASE 24 HOUR 400MG 100CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 42858-0701-01 | RHODES PHARMACEUTICAL | 100 | 400MG | TABLET |
PACKAGE FILES
Generic Name
THEOPHYLLINE
Substance Name
THEOPHYLLINE ANHYDROUS
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA040086
Description
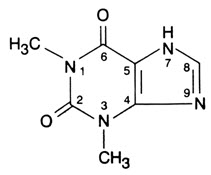
DESCRIPTION Theophylline (Anhydrous) Extended-Release Tablets, in a controlled-release system, allow a 24-hour dosing interval for appropriate patients. Theophylline is structurally classified as a methylxanthine. It occurs as a white, odorless, crystalline powder with a bitter taste. Anhydrous theophylline has the chemical name 1H-Purine-2,6-dione, 3,7-dihydro-1,3-dimethyl-, and is represented by the following structural formula: The molecular formula of anhydrous theophylline is C 7 H 8 N 4 O 2 with a molecular weight of 180.17. Each extended-release tablet for oral administration contains 400 or 600 mg of anhydrous theophylline. Inactive Ingredients: cetostearyl alcohol, hydroxyethyl cellulose, magnesium stearate, povidone, and talc. Chemical Structure
How Supplied
HOW SUPPLIED Theophylline (Anhydrous) Extended-Release Tablets are supplied as follows: 400 mg - round flat faced radius edged embossed (i.e. imprinted) with "P" bisect "F" on the upper side and "U" over "400" on lower side. Bottles of 100 tablets NDC 42858-701-01 600 mg - concave rectangular shaped tablet with "P" bisect "F" engraved (i.e. imprinted) on one side and "U 600" on the other side. Bottles of 100 tablets NDC 42858-702-01 Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Dispense in a tight, light-resistant container.
Indications & Usage
INDICATIONS AND USAGE Theophylline is indicated for the treatment of the symptoms and reversible airflow obstruction associated with chronic asthma and other chronic lung diseases, e.g., emphysema and chronic bronchitis.
Dosage and Administration
DOSAGE AND ADMINISTRATION Theophylline (anhydrous) extended-release tablets 400 or 600 mg can be taken once a day in the morning or evening. It is recommended that theophylline (anhydrous) extended-release tablets be taken with meals. Patients should be advised that if they choose to take theophylline (anhydrous) extended-release tablets with food it should be taken consistently with food and if they take it in a fasted condition it should routinely be taken fasted. It is important that the product whenever dosed be dosed consistently with or without food. Theophylline (anhydrous) extended-release tablets are not to be chewed or crushed because it may lead to a rapid release of theophylline with the potential for toxicity. The scored tablet may be split. Infrequently, patients receiving theophylline (anhydrous) extended-release tablets 400 or 600 mg may pass an intact matrix tablet in the stool or via colostomy. These matrix tablets usually contain little or no residual theophylline. Stabilized patients, 12 years of age or older, who are taking an immediate-release or controlled-release theophylline product may be transferred to once-daily administration of 400 or 600 mg theophylline (anhydrous) extended-release tablets on a mg-for-mg basis. It must be recognized that the peak and trough serum theophylline levels produced by the once-daily dosing may vary from those produced by the previous product and/or regimen. General Considerations The steady-state peak serum theophylline concentration is a function of the dose, the dosing interval, and the rate of theophylline absorption and clearance in the individual patient. Because of marked individual differences in the rate of theophylline clearance, the dose required to achieve a peak serum theophylline concentration in the 10 to 20 mcg/mL range varies fourfold among otherwise similar patients in the absence of factors known to alter theophylline clearance (e.g., 400 to 1600 mg/day in adults <60 years old and 10 to 36 mg/kg/day in children 1 to 9 years old). For a given population, there is no single theophylline dose that will provide both safe and effective serum concentrations for all patients. Administration of the median theophylline dose required to achieve a therapeutic serum theophylline concentration in a given population may result in either sub-therapeutic or potentially toxic serum theophylline concentrations in individual patients. For example, at a dose of 900 mg/d in adults <60 years or 22 mg/kg/d in children 1 to 9 years, the steady-state peak serum theophylline concentration will be <10 mcg/mL in about 30% of patients, 10 to 20 mcg/mL in about 50% and 20 to 30 mcg/mL in about 20% of patients. The dose of theophylline must be individualized on the basis of peak serum theophylline concentration measurements in order to achieve a dose that will provide maximum potential benefit with minimal risk of adverse effects. Transient caffeine-like adverse effects and excessive serum concentrations in slow metabolizers can be avoided in most patients by starting with a sufficiently low dose and slowly increasing the dose, if judged to be clinically indicated, in small increments (see Table V ) . Dose increases should only be made if the previous dosage is well tolerated and at intervals of no less than 3 days to allow serum theophylline concentrations to reach the new steady-state. Dosage adjustment should be guided by serum theophylline concentration measurement ( see PRECAUTIONS, Laboratory Tests and DOSAGE AND ADMINISTRATION, Table VI ) . Healthcare providers should instruct patients and caregivers to discontinue any dosage that causes adverse effects, to withhold the medication until these symptoms are gone, and to then resume therapy at a lower, previously tolerated dosage ( see WARNINGS ) . If the patient's symptoms are well controlled, there are no apparent adverse effects, and no intervening factors that might alter dosage requirements ( see WARNINGS and PRECAUTIONS ) , serum theophylline concentrations should be monitored at 6 month intervals for rapidly growing children and at yearly intervals for all others. In acutely ill patients, serum theophylline concentrations should be monitored at frequent intervals, e.g., every 24 hours. Theophylline distributes poorly into body fat; therefore, mg/kg dose should be calculated on the basis of ideal body weight. Table V contains theophylline dosing titration schema recommended for patients in various age groups and clinical circumstances. Table VI contains recommendations for theophylline dosage adjustment based upon serum theophylline concentrations. Application of these general dosing recommendations to individual patients must take into account the unique clinical characteristics of each patient. In general, these recommendations should serve as the upper limit for dosage adjustments in order to decrease the risk of potentially serious adverse events associated with unexpected large increases in serum theophylline concentration. Table V. Dosing initiation and titration (as anhydrous theophylline). Patients with more rapid metabolism clinically identified by higher than average dose requirements should receive a smaller dose more frequently (every 12 hours) to prevent breakthrough symptoms resulting from low trough concentrations before the next dose. A. Children (12 to 15 years) and adults (16 to 60 years) without risk factors for impaired clearance . Titration Step Children <45 kg Children >45 kg and adults 1. Starting Dosage 12 to 14 mg/kg/day up to a maximum of 300 mg/day admin. QD 300 to 400 mg/day If caffeine-like adverse effects occur, then consideration should be given to a lower dose and titrating the dose more slowly (see ADVERSE REACTIONS ). admin. QD 2. After 3 days, if tolerated, increase dose to: 16 mg/kg/day up to a maximum of 400 mg/day admin. QD 400 to 600 mg/day admin. QD 3. After 3 more days, if tolerated, and if needed increase dose to: 20 mg/kg/day up to a maximum of 600 mg/day admin. QD As with all theophylline products, doses greater than 600 mg should be titrated according to blood level (see Table VI ). B. Patients with risk factors for impaired clearance, the elderly (>60 years), and those in whom it is not feasible to monitor serum theophylline concentrations: In children 12 to 15 years of age, the theophylline dose should not exceed 16 mg/kg/day up to a maximum of 400 mg/day in the presence of risk factors for reduced theophylline clearance (see WARNINGS ) or if it is not feasible to monitor serum theophylline concentrations. In adolescents ≥16 years and adults, including the elderly, the theophylline dose should not exceed 400 mg/day in the presence of risk factors for reduced theophylline clearance (see WARNINGS ) or if it is not feasible to monitor serum theophylline concentrations. Table VI. Dosage adjustment guided by serum theophylline concentration. Peak Serum Concentration Dosage Adjustment ¶Dose reduction and/or serum theophylline concentration measurement is indicated whenever adverse effects are present, physiologic abnormalities that can reduce theophylline clearance occur (e.g. sustained fever), or a drug that interacts with theophylline is added or discontinued (see WARNINGS ). <9.9 mcg/mL If symptoms are not controlled and current dosage is tolerated, increase dose about 25%. Recheck serum concentration after three days for further dosage adjustment. 10 to 14.9 mcg/mL If symptoms are controlled and current dosage is tolerated, maintain dose and recheck serum concentration at 6 to12 month intervals. If symptoms are not controlled and current dosage is tolerated, consider adding additional medication(s) to treatment regimen. 15 to 19.9 mcg/mL Consider 10% decrease in dose to provide greater margin of safety even if current dosage is tolerated. 20 to 24.9 mcg/mL Decrease dose by 25% even if no adverse effects are present. Recheck serum concentration after 3 days to guide further dosage adjustment. 25 to 30 mcg/mL Skip next dose and decrease subsequent doses at least 25% even if no adverse effects are present. Recheck serum concentration after 3 days to guide further dosage adjustment. If symptomatic, consider whether overdose treatment is indicated (see recommendations for chronic overdosage ). >30 mcg/mL Treat overdose as indicated (see recommendations for chronic overdosage ). If theophylline is subsequently resumed, decrease dose by at least 50% and recheck serum concentration after 3 days to guide further dosage adjustment.
