Drug Catalog - Product Detail
TERBUTALINE SULFATE TAB 5 MG 100 CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00115-2622-01 | AMNEAL PHARMACEUTICALS | 100 | 5MG | TABLET |
PACKAGE FILES


Generic Name
Substance Name
Product Type
Route
Application Number
Description
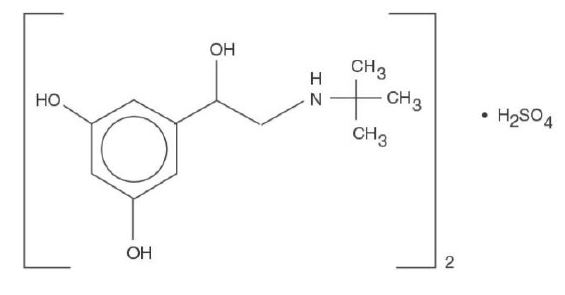
DESCRIPTION Terbutaline sulfate, USP, the active ingredient of terbutaline sulfate tablets, USP is a beta-adrenergic agonist bronchodilator available as tablets of 2.5 mg (2.05 mg of the free base) and 5 mg (4.1 mg of the free base) for oral administration. Terbutaline sulfate is ±-alpha-[( tert -butylamino)methyl]-3,5-dihydroxybenzyl alcohol sulfate (2:1) (salt). The empirical formula is (C 12 H 19 NO 3 ) 2 H 2 SO 4 and the structural formula is Terbutaline sulfate, USP is a white to gray-white crystalline powder. It is odorless or has a faint odor of acetic acid. It is soluble in water and in 0.1N hydrochloric acid, slightly soluble in methanol, and insoluble in chloroform. Its molecular weight is 548.65. Inactive Ingredients. Hydroxypropyl methylcellulose, microcrystalline cellulose, anhydrous lactose, magnesium stearate, povidone, and pregelatinized starch. 1
How Supplied
HOW SUPPLIED Terbutaline sulfate tablets, USP are packaged in bottles of 100 and 500 tablets. Descriptions of the 2.5 mg and 5 mg tablets follow: Tablets 2.5 mg –Each off-white, oval, convex tablet debossed with "G" on one side and "2611" on the other side Bottles of 100: NDC 0115-2611-01 Bottles of 500: NDC 0115-2611-02 Tablets 5 mg –Each off-white, round, convex tablet debossed with "G" on one side and "2622" on the other side Bottles of 100: NDC 0115-2622-01 Bottles of 500: NDC 0115-2622-02 Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Dispense in tightly-closed, light-resistant container (USP).
Indications & Usage
INDICATIONS AND USAGE Terbutaline sulfate tablets are indicated for the prevention and reversal of bronchospasm in patients 12 years of age and older with asthma and reversible bronchospasm associated with bronchitis and emphysema.
Dosage and Administration
DOSAGE AND ADMINISTRATION Adults The usual oral dose of terbutaline sulfate for adults is 5 mg administered at approximately six-hour intervals, three times daily, during the hours the patient is usually awake. If side effects are particularly disturbing, the dose may be reduced to 2.5 mg three times daily, and still provide a clinically significant improvement in pulmonary function. The total dose within 24 hours should not exceed 15 mg. Children Terbutaline sulfate is not recommended for use in children below the age of 12 years. A dosage of 2.5 mg three times daily is recommended for children 12 to 15 years of age. The total dose within 24 hours should not exceed 7.5 mg. If a previously effective dosage regimen fails to provide the usual relief, medical advice should be sought immediately as this is often a sign of seriously worsening asthma that would require reassessment of therapy.
