Drug Catalog - Product Detail
TENOFOVIR DISPOROXIL FUMARATE 300MG TB 30CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 69097-0533-02 | CIPLA USA | 30 | 300MG | TABLET |
PACKAGE FILES

Generic Name
TENOFOVIR DISOPROXIL FUMARATE
Substance Name
TENOFOVIR DISOPROXIL FUMARATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA078800
Description
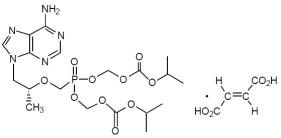
11 DESCRIPTION Tenofovir disoproxil fumarate (a prodrug of tenofovir) is a fumaric acid salt of bis-isopropoxycarbonyloxymethyl ester derivative of tenofovir. TDF is converted in vivo to tenofovir, an acyclic nucleoside phosphonate (nucleotide) analog of adenosine 5'-monophosphate. Tenofovir exhibits activity against HIV-1 reverse transcriptase. The chemical name of TDF is 9-[( R )-2-[[bis[[(isopropoxycarbonyl)oxy]methoxy]phosphinyl]methoxy]propyl]adenine fumarate (1:1). It has a molecular formula of C 19 H 30 N 5 O 10 P • C 4 H 4 O 4 and a molecular weight of 635.52. It has the following structural formula: Tenofovir disoproxil fumarate is a white to off-white crystalline powder with a solubility of 13.4 mg/mL in distilled water at 25°C. It has an octanol/phosphate buffer (pH 6.5) partition coefficient (log p) of 1.25 at 25°C. Tenofovir disoproxil fumarate tablets are for oral administration. Each tablet contains 300 mg of tenofovir disoproxil fumarate, which is equivalent to 245 mg of tenofovir disoproxil, and the following inactive ingredients: Lactose monohydrate, Croscarmellose sodium, Corn starch, Polysorbate 80, Microcrystalline cellulose, Magnesium stearate, Opadry II Y-30-10671-A Light blue. (Lactose monohydrate, HPMC2910/Hypromellose 15cP, Titanium dioxide, Triacetin / Glycerol triacetate, FD&C Blue #2/Indigo carmine Aluminum Lake). In this insert, all dosages are expressed in terms of TDF except where otherwise noted. Image
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING The light blue capsule shaped, biconvex film coated tablets contain 300 mg of tenofovir disoproxil fumarate, which is equivalent to 245 mg of tenofovir disoproxil, are debossed with "C533" on one side and plain on other side. Each bottle contains 30 tablets, three desiccants (silica gel), closed with a child resistant closure (NDC 69097-533-02) 1000 tablets, a desiccant (silica gel), Rayon sanicoil and closed with a non child resistant closure (NDC 69097-533-15) Store at 25 °C (77 °F), excursions permitted between 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]. Keep the bottle tightly closed. Dispense only in original container. Do not use if seal over bottle opening is broken or missing.
Indications & Usage
1 INDICATIONS AND USAGE Tenofovir disoproxil fumarate is a nucleotide analog HIV-1 reverse transcriptase inhibitor and an HBV reverse transcriptase inhibitor and is indicated: in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults and pediatric patients 2 years of age and older weighing at least 10 kg. ( 1.1 ) for the treatment of chronic hepatitis B in adults and pediatric patients 12 years and older. ( 1.2 ) 1.1 HIV-1 Infection Tenofovir disoproxil fumarate is indicated in combination with other antiretroviral agents for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in adults and pediatric patients 2 years of age and older weighing at least 10 kg. 1.2 Chronic Hepatitis B Tenofovir disoproxil fumarate is indicated for the treatment of chronic hepatitis B virus (HBV) in adults and pediatric patients 12 years of age and older. Pediatric use information is approved for Gilead Sciences, Inc.'s VIREAD® (tenofovir disoproxil fumarate) tablets. However, due to Gilead Sciences, Inc.'s marketing exclusivity rights, this drug product is not labeled with that pediatric information
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Testing: Prior to or when initiating tenofovir disoproxil fumarate test for hepatitis B virus infection and HIV-1 infection. Prior to initiation and during use of tenofovir disoproxil fumarate, on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose, and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorous. ( 2.1 ) Recommended tablet dosage in adults and pediatric patients weighing at least 35 kg: One tenofovir disoproxil fumarate tablet 300 mg tablet once daily taken orally without regard to food. ( 2.2 ) Recommended dosage in pediatric patients at least 2 years of age and adults: Tablets: For patients weighing at least 17 kg who can swallow an intact tablet, one tenofovir disoproxil fumarate tablet (150 mg, 200 mg, 250 mg, or 300 mg based on body weight) once daily taken orally without regard to food. ( 2.2 ) Recommended dosage in renally impaired adult patients: Creatinine clearance (CrCl) 30-49 mL/min: 300 mg every 48 hours. ( 2.4 ) CrCl 10-29 mL/min: 300 mg every 72 to 96 hours. ( 2.4 ) Hemodialysis: 300 mg every 7 days or after approximately 12 hours of dialysis. ( 2.4 ) 2.1 Testing Prior to Initiation of Tenofovir Disoproxil Fumarate for Treatment of HIV-1 Infection or Chronic Hepatitis B Prior to or when initiating tenofovir disoproxil fumarate tablets, test patients for HBV infection and HIV-1 infection. Tenofovir disoproxil fumarate alone should not be used in patients with HIV-1 infection [see Warnings and Precautions ( 5.3 )] . Prior to initiation and during use of tenofovir disoproxil fumarate tablets, on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus [see Warnings and Precautions ( 5.2 )] . 2.2 Recommended Tablet Dosage in Adults and Pediatric Patients 2 Years and Older Weighing at Least 17 kg The recommended dosage of tenofovir disoproxil fumarate tablets in adults and pediatric patients weighing at least 35 kg is one 300 mg tablet taken orally once daily without regard to food . The dosage for tenofovir disoproxil fumarate is the same for both HIV and HBV indications. The recommended dosage of tenofovir disoproxil fumarate tablet in adults and pediatric patients 2 years and older weighing at least 17 kg is 8 mg of tenofovir disoproxil fumarate (TDF) per kg of body weight (up to a maximum of 300 mg) once daily. Dosage for pediatric patients 2 years and older weighing between 17 kg and 35 kg and able to swallow an intact tablet is provided in Table 1. Weight should be monitored periodically and the tenofovir disoproxil fumarate dose adjusted accordingly. Table 1 Recommended Dosing for Patients 2 Years and Older and Weighing at Least 17 kg Using Tenofovir Disoproxil Fumarate Tablets Body Weight (kg) Dosing of Tenofovir Disoproxil fumarate Tablets 17 to less than 22 one 150 mg tablet once daily 22 to less than 28 one 200 mg tablet once daily 28 to less than 35 one 250 mg tablet once daily at least 35 one 300 mg tablet once daily 2.4 Dosage Adjustment in Patients with Renal Impairment Significant increase in drug exposures occurred when tenofovir disoproxil fumarate was administered to subjects with moderate to severe renal impairment (creatinine clearance below 50 mL/min). Table 3 provides dosage interval adjustment for patients with renal impairment. No dosage adjustment of tenofovir disoproxil fumarate tablets 300 mg is necessary for patients with mild renal impairment (creatinine clearance 50–80 mL/min) [see Warnings and Precautions ( 5.3 ), Use in Specific Populations ( 8.6 ), and Clinical Pharmacology ( 12.3 )] . Table 3 Dosage Interval Adjustment for Adult Patients with Altered Creatinine Clearance a. Calculated using ideal (lean) body weight. b. Generally once weekly assuming 3 hemodialysis sessions a week of approximately 4 hours' duration. Tenofovir disoproxil fumarate should be administered following completion of dialysis. Creatinine Clearance (mL/min) a Hemodialysis Patients 50 or greater 30-49 10-29 Recommended 300 mg Dosing Interval Every 24 hours Every 48 hours Every 72 to 96 hours Every 7 days or after a total of approximately 12 hours of dialysis b No data are available to make dosage recommendations in patients with creatinine clearance below 10 mL/min who are not on hemodialysis. No data are available to make dosage recommendations in pediatric patients with renal impairment.
