Drug Catalog - Product Detail
TEMOZOLOMIDE 5MG 3x5 Blister CAP
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 47335-0890-72 | SUN PHARMACEUTICALS | 15 | 5MG | NA |
PACKAGE FILES






Generic Name
TEMOZOLOMIDE
Substance Name
TEMOZOLOMIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA201742
Description
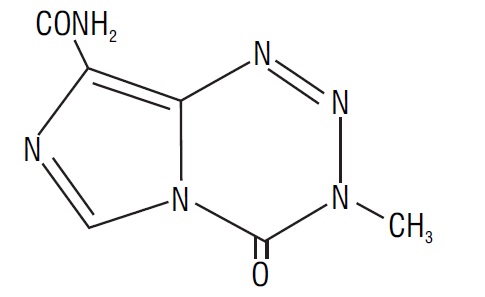
11 DESCRIPTION Temozolomide is an alkylating drug. The chemical name of temozolomide is 3,4-dihydro-3-methyl-4-oxoimidazo[5,1-d]- as -tetrazine-8-carboxamide. The structural formula of temozolomide is: The material is a white to light tan or light pink powder with a molecular formula of C 6 H 6 N 6 O 2 and a molecular weight of 194.15. The molecule is stable at acidic pH (<5) and labile at pH >7; hence temozolomide capsules, USP can be administered orally. The prodrug, temozolomide, is rapidly hydrolyzed to the active 5-(3-methyltriazen-1-yl) imidazole-4-carboxamide (MTIC) at neutral and alkaline pH values, with hydrolysis taking place even faster at alkaline pH. Each capsule for oral use contains either 5 mg, 20 mg, 100 mg, 140 mg, 180 mg, or 250 mg of temozolomide, USP. The inactive ingredients for temozolomide capsules, USP are: lactose anhydrous, sodium starch glycolate, tartaric acid, and stearic acid. The capsule shell contains gelatin, titanium dioxide, and sodium lauryl sulfate. The imprinting ink contains shellac, dehydrated alcohol, butyl alcohol, propylene glycol, strong ammonia solution, FD&C Blue # 1 Aluminum Lake (5 mg, 140 mg), yellow iron oxide (5 mg, 20 mg, 100 mg), red iron oxide (100 mg, 180 mg), titanium dioxide (100 mg, 140 mg), potassium hydroxide (100 mg, 250 mg) and black iron oxide (250 mg). spl-temozolomide-structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Temozolomide is a hazardous drug. Follow applicable special handling and disposal procedures.1 Temozolomide capsules, USP are supplied in amber glass bottles with child-resistant caps or child-resistant blister packs containing the following capsule strengths: 5 mg hard gelatin capsules have white opaque caps and bodies, imprinted with band and the dosage strength “5 mg” on body and “890” on cap in green ink, containing white to light tan/light pink powder and are supplied as: Bottles of 5 with Child Resistant Cap…….………..NDC 47335-890-80 Bottles of 14 with Child Resistant Cap………….....NDC 47335-890-21 Bottles of 20 with Child Resistant Cap…………….NDC 47335-890-87 Unit-dose blister pack of 5 (1 × 5).............................NDC 47335-890-74 Unit-dose blister pack of 15 (3 x 5)...........................NDC 47335-890-72 Unit-dose blister pack of 20 (4 × 5)...........................NDC 47335-890-75 20 mg hard gelatin capsules have white opaque caps and bodies, imprinted with band and the dosage strength “20 mg” on body and “891” on cap in yellow ink, containing white to light tan/light pink powder and are supplied as: Bottles of 5 with Child Resistant Cap…….………..NDC 47335-891-80 Bottles of 14 with Child Resistant Cap………….....NDC 47335-891-21 Bottles of 20 with Child Resistant Cap…………….NDC 47335-891-87 Unit-dose blister pack of 5 (1 × 5) ...........................NDC 47335-891-74 Unit-dose blister pack of 15 (3 x 5)..........................NDC 47335-891-72 Unit-dose blister pack of 20 (4 × 5) .........................NDC 47335-891-75 100 mg hard gelatin capsules have white opaque caps and bodies, imprinted with band and the dosage strength “100 mg” on body and “892” on cap in pink ink, containing white to light tan/light pink powder and are supplied as: Bottles of 5 with Child Resistant Cap…….………..NDC 47335-892-80 Bottles of 14 with Child Resistant Cap………….....NDC 47335-892-21 Bottles of 20 with Child Resistant Cap…………….NDC 47335-892-87 Unit-dose blister pack of 5 (1 × 5) ...........................NDC 47335-892-74 Unit-dose blister pack of 15 (3 x 5)..........................NDC 47335-892-72 Unit-dose blister pack of 20 (4 × 5)..........................NDC 47335-892-75 140 mg hard gelatin capsules have white opaque caps and bodies, imprinted with band and the dosage strength “140 mg” on body and “929” on cap in blue ink, containing white to light tan/light pink powder and are supplied as: Bottles of 5 with Child Resistant Cap…….………..NDC 47335-929-80 Bottles of 14 with Child Resistant Cap………….....NDC 47335-929-21 Bottles of 20 with Child Resistant Cap…………….NDC 47335-929-87 Unit-dose blister pack of 5 (1 × 5) ...........................NDC 47335-929-74 Unit-dose blister pack of 15 (3 x 5)..........................NDC 47335-929-72 Unit-dose blister pack of 20 (4 × 5)..........................NDC 47335-929-75 180 mg hard gelatin capsules have white opaque caps and bodies, imprinted with band and the dosage strength “180 mg” on body and “930” on cap in reddish brown ink, containing white to light tan/light pink powder and are supplied as: Bottles of 5 with Child Resistant Cap…….………..NDC 47335-930-80 Bottles of 14 with Child Resistant Cap………….... NDC 47335-930-21 Bottles of 20 with Child Resistant Cap…………….NDC 47335-930-87 Unit-dose blister pack of 5 (1 × 5) ...........................NDC 47335-930-74 Unit-dose blister pack of 15 (3 x 5)..........................NDC 47335-930-72 Unit-dose blister pack of 20 (4 × 5) .........................NDC 47335-930-75 250 mg hard gelatin capsules have white opaque caps and bodies, imprinted with band and the dosage strength “250 mg” on body and “893” on cap in black ink, containing white to light tan/light pink powder and are supplied as: Bottles of 5 with Child Resistant Cap……................NDC 47335-893-80 Bottles of 14 with Child Resistant Cap…………......NDC 47335-893-21 Bottles of 20 with Child Resistant Cap……………..NDC 47335-893-87 Unit-dose blister pack of 5 (1 × 5) ............................NDC 47335-893-74 Unit-dose blister pack of 20 (4 × 5)...........................NDC 47335-893-75 Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° and 30°C (59° and 86°F) [see USP Controlled Room Temperature]. Dispense in tight, light-resistant containers as defined in USP/NF or retain in original bottle.
Indications & Usage
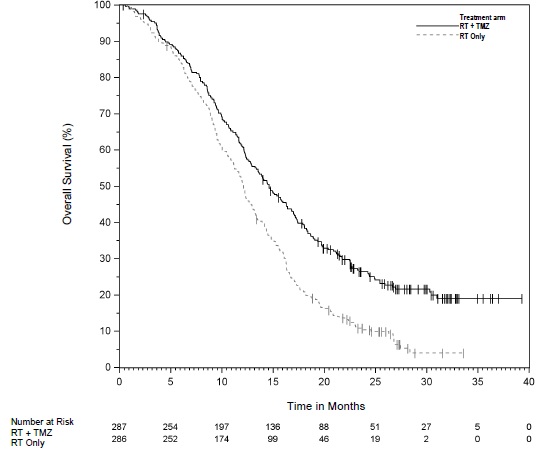
1 INDICATIONS AND USAGE Temozolomide is an alkylating drug indicated for the treatment of adults with: • Newly diagnosed glioblastoma concomitantly with radiotherapy and then as maintenance treatment. (1.1) • Anaplastic astrocytoma. (1.2) o Adjuvant treatment of adults with newly diagnosed anaplastic astrocytoma. (1.2) o Treatment of adults with refractory anaplastic astrocytoma. (1.2) 1.1 Newly Diagnosed Glioblastoma Temozolomide capsules are indicated for the treatment of adults with newly diagnosed glioblastoma, concomitantly with radiotherapy and then as maintenance treatment. 1.2 Anaplastic Astrocytoma Temozolomide capsules are indicated for the: • adjuvant treatment of adults with newly diagnosed anaplastic astrocytoma; • treatment of adults with refractory anaplastic astrocytoma.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Administer orally. (2.4) Newly Diagnosed Glioblastoma: o 75 mg/m2 once daily for 42 to 49 days concomitant with focal radiotherapy followed by initial maintenance dose of 150 mg/m2 once daily for Days 1 to 5 of each 28-day cycle for 6 cycles. May increase maintenance dose to 200 mg/m2 for Cycles 2 to 6 based on toxicity. (2.1) o Provide Pneumocystis pneumonia (PCP) prophylaxis during concomitant phase and continue in patients who develop lymphopenia until resolution to Grade 1 or less. (2.1) Adjuvant Treatment of Newly Diagnosed Anaplastic Astrocytoma: Beginning 4 weeks after the end of radiotherapy, administer temozolomide orally in a single dose on days 1 to 5 of a 28-day cycle for 12 cycles. The recommended dosage for Cycle 1 is 150 mg/m2 per day and for Cycles 2 to 12 is 200 mg/m2 if patient experienced no or minimal toxicity in Cycle 1. (2.2) Refractory Anaplastic Astrocytoma: Initial dose of 150 mg/m2 once daily on Days 1 to 5 of each 28-day cycle. (2.2) 2.1 Monitoring to Inform Dosage and Administration Prior to dosing, withhold temozolomide capsules until patients have an absolute neutrophil count (ANC) of 1.5 x 10 9 /L or greater and a platelet count of 100 x 10 9 /L or greater. For concomitant radiotherapy, obtain a complete blood count prior to initiation of treatment and weekly during treatment. For the 28-day treatment cycles, obtain a complete blood count prior to treatment on Day 1 and on Day 22 of each cycle. Perform complete blood counts weekly until recovery if the ANC falls below 1.5 x 10 9 /L and the platelet count falls below 100 x 10 9 /L. For concomitant use with focal radiotherapy, obtain a complete blood count weekly and as clinically indicated. 2.2 Recommended Dosage and Dosage Modifications for Newly Diagnosed Glioblastoma Administer temozolomide capsules once daily for 42 to 49 consecutive days during the concomitant use phase with focal radiotherapy, and then once daily on Days 1 to 5 of each 28-day cycle for 6 cycles during the maintenance use phase. Provide Pneumocystis pneumonia (PCP) prophylaxis during the concomitant use phase and continue in patients who develop lymphopenia until resolution to Grade 1 or less [see Warnings and Precautions (5.3)]. Concomitant Use Phase: The recommended dosage of temozolomide capsules is 75 mg/m2 once daily for 42 to 49 days in combination with focal radiotherapy. Focal radiotherapy includes the tumor bed or resection site with a 2 to 3 cm margin. Other administration schedules have been used. Obtain a complete blood count weekly. The recommended dosage modifications due to adverse reactions during concomitant use phase are provided in Table 1. TABLE 1: Dosage Modifications Due to Adverse Reactions During Concomitant Use Phase Adverse Reaction Interruption Discontinuation Absolute Neutrophil Count Withhold temozolomide capsules if ANC is greater than or equal to 0.5 x 10 9 /L and less than 1.5 x 10 9 /L. Resume temozolomide capsules at the same dose when ANC is greater than or equal to 1.5 x 109/L. Discontinue temozolomide capsules if ANC is less than 0.5 × 10 9 /L Platelet Count Withhold temozolomide capsules if platelet count is greater than or equal to 10 x 10 9 /L and less than 100 x 10 9 /L. Resume temozolomide capsules at the same dose when platelet count is greater than or equal to 100 x 109/L. Discontinue temozolomide capsules if platelet count is less than 10 × 10 9 /L Non-hematological Adverse Reaction (except for alopecia, nausea, vomiting) Withhold temozolomide capsules if Grade 2 adverse reaction occurs. Resume temozolomide capsules at the same dose when resolution to Grade 1 or less. Discontinue temozolomide capsules if Grade 3 or 4 adverse reaction occurs. Single Agent Maintenance Use Phase: Beginning 4 weeks after concomitant use phase completion, administer temozolomide capsules once daily on Days 1 to 5 of each 28-day cycle for 6 cycles. The recommended dosage of temozolomide capsules in the maintenance use phase is: • Cycle 1: 150 mg/m2 per day on days 1 to 5. • Cycles 2 to 6: May increase to 200 mg/m2 per day on days 1 to 5 before starting Cycle 2 if no dosage interruptions or discontinuations are required (Table 1). If the dose is not escalated at the onset of Cycle 2, do not increase the dose for Cycles 3 to 6. Obtain a complete blood count on Day 22 and then weekly until the ANC is above 1.5 x 109/L and the platelet count is above 100 x 109/L. Do not start the next cycle until the ANC and platelet count exceed these levels. The recommended dosage modifications due to adverse reactions during the maintenance use phase are provided in Table 2. If temozolomide capsules are withheld, reduce the dose for the next cycle by 50 mg/m2 per day. Permanently discontinue temozolomide capsules in patients who are unable to tolerate a dose of 100 mg/m2 per day. TABLE 2: Dosage Modifications Due to Adverse Reactions During Maintenance and Adjuvant Treatment Adverse Reactions Interruption and Dose Reduction Discontinuation Absolute Neutrophil Count Withhold temozolomide capsules if ANC less than 1 x 10 9 /L. When ANC is above 1.5 x 10 9 /L, resume temozolomide capsules at reduced dose for the next cycle. Discontinue temozolomide capsules if unable to tolerate a dose of 100 mg/m2 per day. Platelet Count Withhold temozolomide capsules if platelet less than 50 x 10 9 /L. When platelet count is above 100 x 10 9 /L, resume temozolomide capsules at reduced dose for the next cycle. Discontinue temozolomide capsules if unable to tolerate a dose of 100 mg/m2 per day. Non-hematological Adverse Reactions (except for alopecia, nausea, vomiting) Withhold temozolomide capsules if Grade 3 adverse reaction occurs. When resolved to Grade 1 or less, resume temozolomide capsules at reduced dose for the next cycle. Discontinue temozolomide capsules if recurrent Grade 3 adverse reaction occurs after dose reduction, if Grade 4 adverse reaction occurs, or if unable to tolerate a dose of 100 mg/m2 per day. 2.3 Recommended Dosage and Dosage Modifications for Anaplastic Astrocytoma Adjuvant Treatment of Newly Diagnosed Anaplastic Astrocytoma Beginning 4 weeks after the end of radiotherapy, administer temozolomide capsules orally in a single dose on days 1 to 5 of a 28-day cycle for 12 cycles. The recommended dosage of temozolomide capsules is: • Cycle 1: 150 mg/m 2 per day on days 1 to 5. • Cycles 2 to 12: 200 mg/m 2 per day on days 1 to 5 if patient experienced no or minimal toxicity in Cycle 1. If the dose was not escalated at the onset of Cycle 2, do not increase the dose during Cycles 3 to 6. The recommended complete blood count testing and dosage modifications due to adverse reactions during adjuvant treatment are provided above and in Table 2 [see Dosage and Administration (2.2)]. Refractory Anaplastic Astrocytoma The recommended initial dosage of temozolomide capsules are 150 mg/m 2 once daily on Days 1 to 5 of each 28-day cycle. Increase the temozolomide dose to 200 mg/m 2 per day if the following conditions are met at the nadir and on Day 1 of the next cycle: ANC is greater than or equal to 1.5 x 10 9 /L, and Platelet count is greater than or equal to 100 x 10 9 /L. Continue temozolomide capsules until disease progression or unacceptable toxicity. Obtain a complete blood count on Day 22 and then weekly until the ANC is above 1.5 x 10 9 /L and the platelet count is above 100 x 10 9 /L. Do not start the next cycle until the ANC and platelet count exceed these levels. If the ANC is less than 1 x 10 9 /L or the platelet count is less than 50 x 10 9 /L during any cycle, reduce the temozolomide dose for the next cycle by 50 mg/m 2 per day. Permanently discontinue temozolomide capsules in patients who are unable to tolerate a dose of 100 mg/m 2 per day. 2.4 Preparation and Administration Temozolomide is a hazardous drug. Follow applicable special handling and disposal procedures. 1 Temozolomide capsules Take temozolomide capsules at the same time each day. Administer temozolomide capsules consistently with respect to food (fasting vs. nonfasting) [see Clinical Pharmacology (12.3)]. To reduce nausea and vomiting, take temozolomide capsules on an empty stomach or at bedtime and consider antiemetic therapy prior to and following temozolomide capsule administration. Swallow temozolomide capsules whole with water. Advise patients not to open, chew, or dissolve the contents of the capsules [see Warnings and Precautions (5.6)]. If capsules are accidentally opened or damaged, take precautions to avoid inhalation or contact with the skin or mucous membranes. In case of powder contact, wash the affected area with water immediately.
