Drug Catalog - Product Detail
TAMSULOSIN HCL CAP 0.4MG 500CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65862-0598-05 | AUROBINDO PHARMA | 500 | 0.4MG | CAPSULE |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
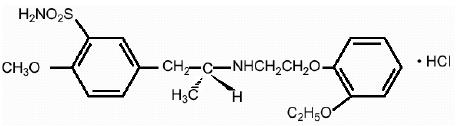
11 DESCRIPTION Tamsulosin hydrochloride is an antagonist of alpha 1A adrenoceptors in the prostate. Tamsulosin hydrochloride is (-)-( R )-5-[2-[[2-( o -Ethoxyphenoxy) ethyl]amino]propyl]-2-methoxybenzenesulfonamide, monohydrochloride. Tamsulosin hydrochloride USP is a white or almost white crystalline powder that melts with decomposition at approximately 230°C. It is sparingly soluble in water and methanol, slightly soluble in glacial acetic acid and ethanol, and practically insoluble in ether. The molecular formula of tamsulosin hydrochloride is C 20 H 28 N 2 O 5 S • HCl. The molecular weight of tamsulosin hydrochloride is 444.98. Its structural formula is: Each tamsulosin hydrochloride capsule, USP for oral administration contains tamsulosin hydrochloride USP 0.4 mg, and the following inactive ingredients: calcium stearate, FD&C Blue 2, gelatin, iron oxide red, iron oxide yellow, microcrystalline cellulose, methacrylic acid copolymer dispersion, sodium lauryl sulfate, talc, triacetin, and titanium dioxide. The capsules are printed with SW-9008 Black Ink containing black iron oxide, butyl alcohol, dehydrated alcohol, isopropyl alcohol, potassium hydroxide, propylene glycol, shellac, and strong ammonia solution. Meets USP dissolution test 10. Chemical Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Tamsulosin Hydrochloride Capsules USP, 0.4 mg are olive green opaque/orange opaque size ‘0’ hard gelatin capsules imprinted with ‘D’ on cap and ‘53’ on body with black edible ink filled with white to off-white beadlets. Bottles of 100 NDC 65862-598-01 Bottles of 500 NDC 65862-598-05 3 x 10 Unit-dose Capsules NDC 65862-598-10 Blister Pack of 30 Capsules NDC 58118-0598-8 Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Preserve in tight container. Avoid excessive moisture. Keep tamsulosin hydrochloride capsules and all medicines out of reach of children.
Indications & Usage
1 INDICATIONS AND USAGE Tamsulosin hydrochloride capsules are indicated for the treatment of the signs and symptoms of benign prostatic hyperplasia (BPH) [see Clinical Studies (14) ] . Tamsulosin hydrochloride capsules are not indicated for the treatment of hypertension. Tamsulosin hydrochloride capsules are an alpha 1 adrenoceptor antagonist indicated for treatment of the signs and symptoms of benign prostatic hyperplasia (1) Tamsulosin hydrochloride capsules are not indicated for the treatment of hypertension (1)
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Tamsulosin hydrochloride capsules 0.4 mg once daily is recommended as the dose for the treatment of the signs and symptoms of BPH. It should be administered approximately one-half hour following the same meal each day. Tamsulosin hydrochloride capsules should not be crushed, chewed or opened. For those patients who fail to respond to the 0.4 mg dose after 2 to 4 weeks of dosing, the dose of tamsulosin hydrochloride capsules can be increased to 0.8 mg once daily. Tamsulosin hydrochloride capsules 0.4 mg should not be used in combination with strong inhibitors of CYP3A4 (e.g., ketoconazole) [see Warnings and Precautions (5.2) ] . If tamsulosin hydrochloride capsules administration is discontinued or interrupted for several days at either the 0.4 mg or 0.8 mg dose, therapy should be started again with the 0.4 mg once-daily dose. 0.4 mg once daily taken approximately one-half hour following the same meal each day. Tamsulosin hydrochloride capsules should not be crushed, chewed or opened. ( 2 ) Can be increased to 0.8 mg once daily for patients who fail to respond to the 0.4 mg dose after 2 to 4 weeks of dosing (2) If discontinued or interrupted for several days, therapy should start again with the 0.4 mg once-daily dose (2)
