Drug Catalog - Product Detail
TADALAFIL TABS USP 20MG 60CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 69097-0526-03 | CIPLA USA | 60 | 20MG | TABLET |
PACKAGE FILES
Generic Name
TADALAFIL
Substance Name
TADALAFIL
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA210255
Description
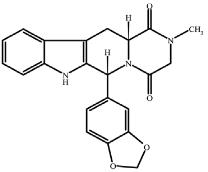
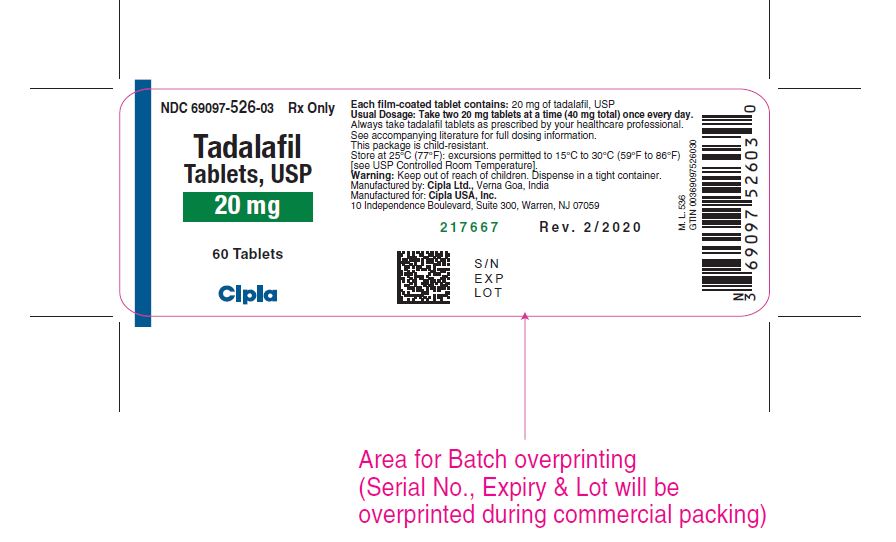
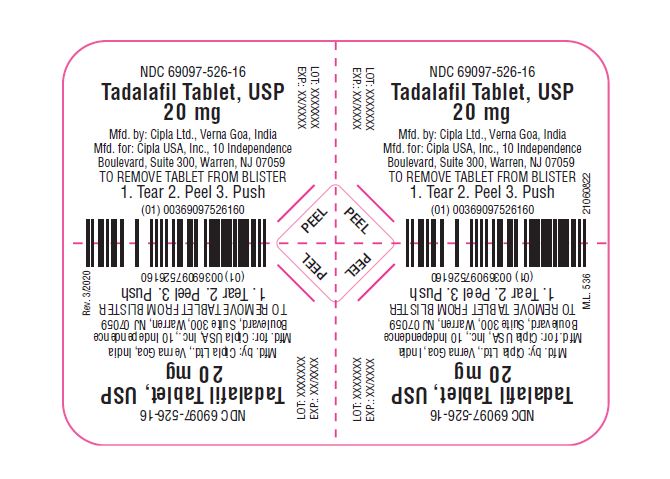
11 DESCRIPTION Tadalafil tablets, an oral treatment for pulmonary arterial hypertension, is a selective inhibitor of cyclic guanosine monophosphate (cGMP)–specific phosphodiesterase type 5 (PDE5). Tadalafil has the empirical formula C 22 H 19 N 3 O 4 representing a molecular weight of 389.41. The structural formula is: The chemical designation is pyrazino[1´,2´:1,6]pyrido[3,4–b]indole-1,4-dione, 6-(1,3-benzodioxol-5-yl)2,3,6,7,12,12a- hexahydro-2-methyl-, (6R,12aR)-. It is a crystalline solid that is practically insoluble in water and very slightly soluble in ethanol. Tadalafil tablets, USP 20 mg are available brown colored, capsule shaped, biconvex film coated tablet with 'Cipla' debossed on one side and 526 on other side. Each tablet contains 20 mg of tadalafil and the following inactive ingredients: : croscarmellose sodium, hydroxypropyl cellulose, hypromellose, iron oxide red, iron oxide yellow, lactose monohydrate, magnesium stearate, microcrystalline cellulose, sodium lauryl sulfate, titanium dioxide and triacetin. Image
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied Tadalafil tablets, USP are supplied as follows: Brown colored, capsule shaped, biconvex film coated tablet with 'Cipla' debossed on one side and 526 on other side. Carton of 4 Tablets (1 x 4 Unit-dose) with child-resistant blister NDC 69097-526-16 Bottles of 60 with child-resistant closure NDC 69097-526-03 16.2 Storage Store at 25°C (77°F): excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature.] Keep out of reach of children.
Indications & Usage
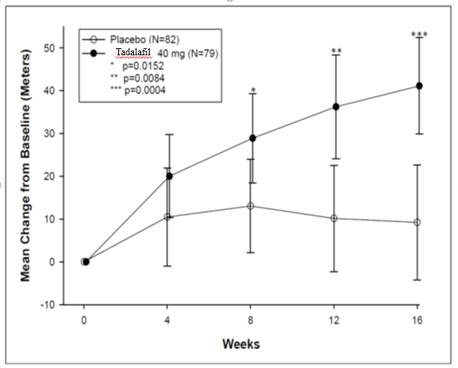
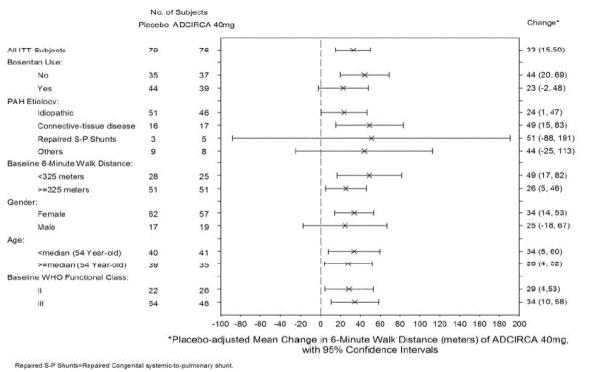
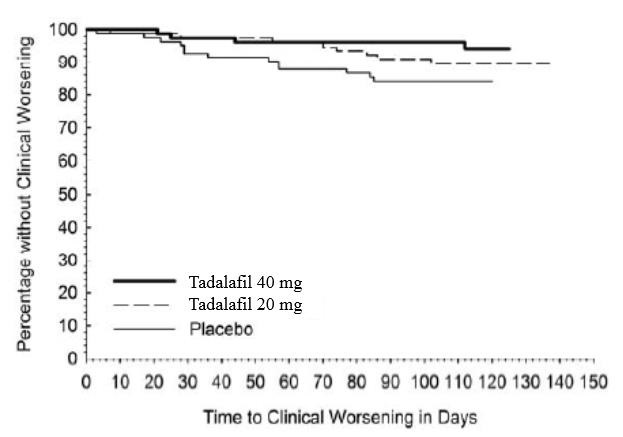
1 INDICATIONS AND USAGE Tadalafil is a phosphodiesterase 5 (PDE5) inhibitor indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1) to improve exercise ability. Studies establishing effectiveness included predominately patients with NYHA Functional Class II – III symptoms and etiologies of idiopathic or heritable PAH (61%) or PAH associated with connective tissue diseases (23%). ( 1.1 ) 1.1 Pulmonary Arterial Hypertension Tadalafil tablets are indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1) to improve exercise ability. Studies establishing effectiveness included predominately patients with NYHA Functional Class II – III symptoms and etiologies of idiopathic or heritable PAH (61%) or PAH associated with connective tissue diseases (23%).
Dosage and Administration
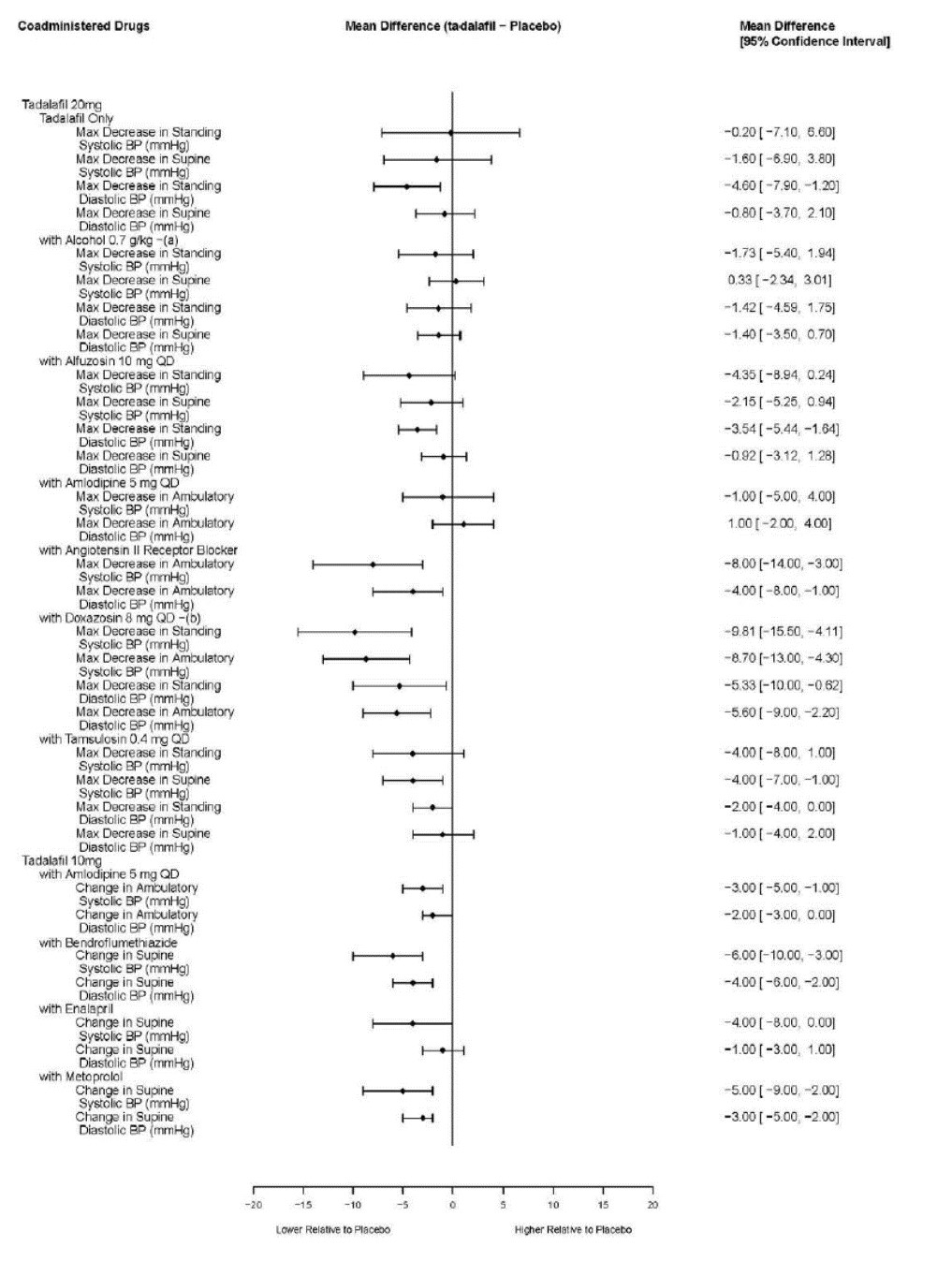
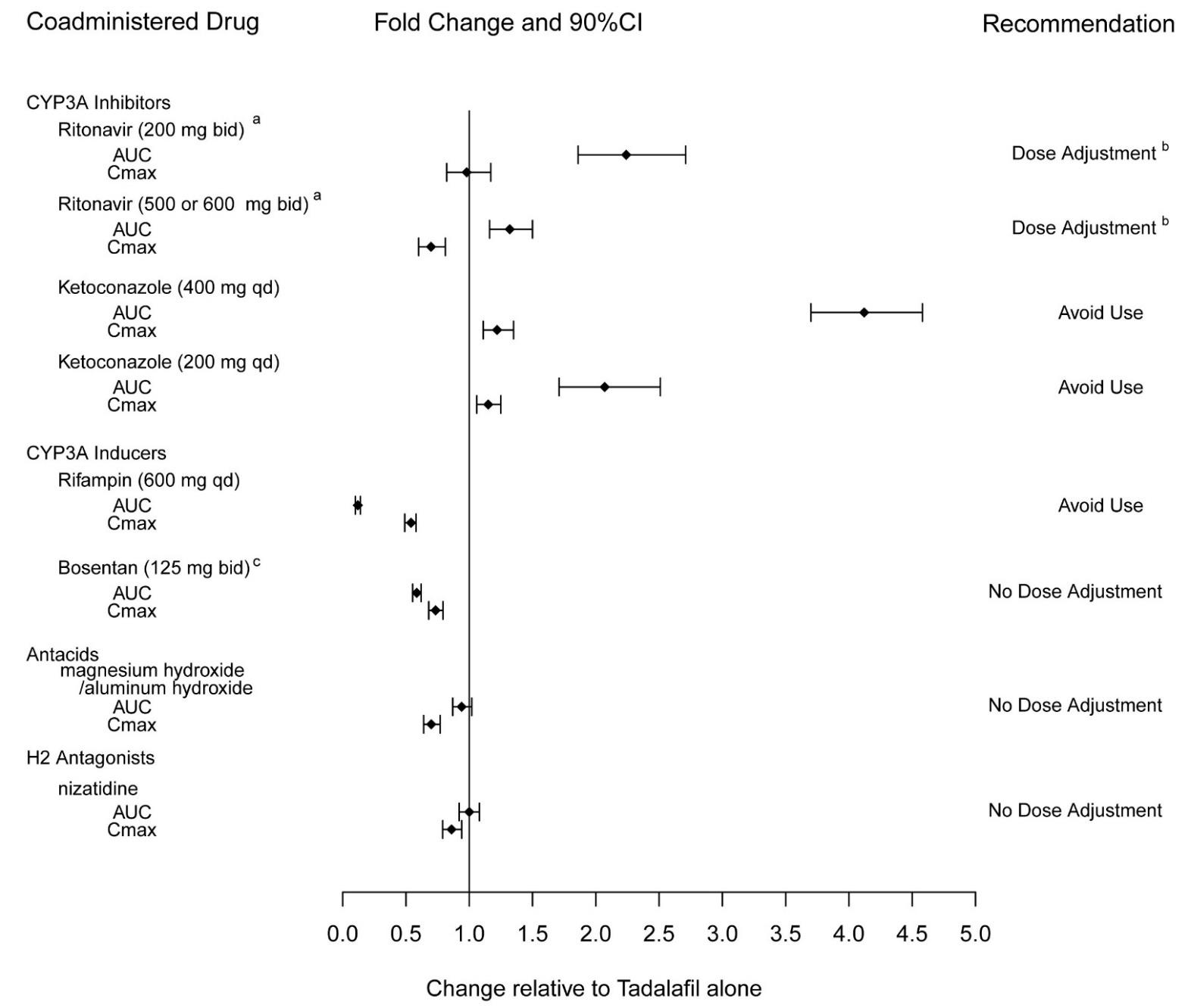
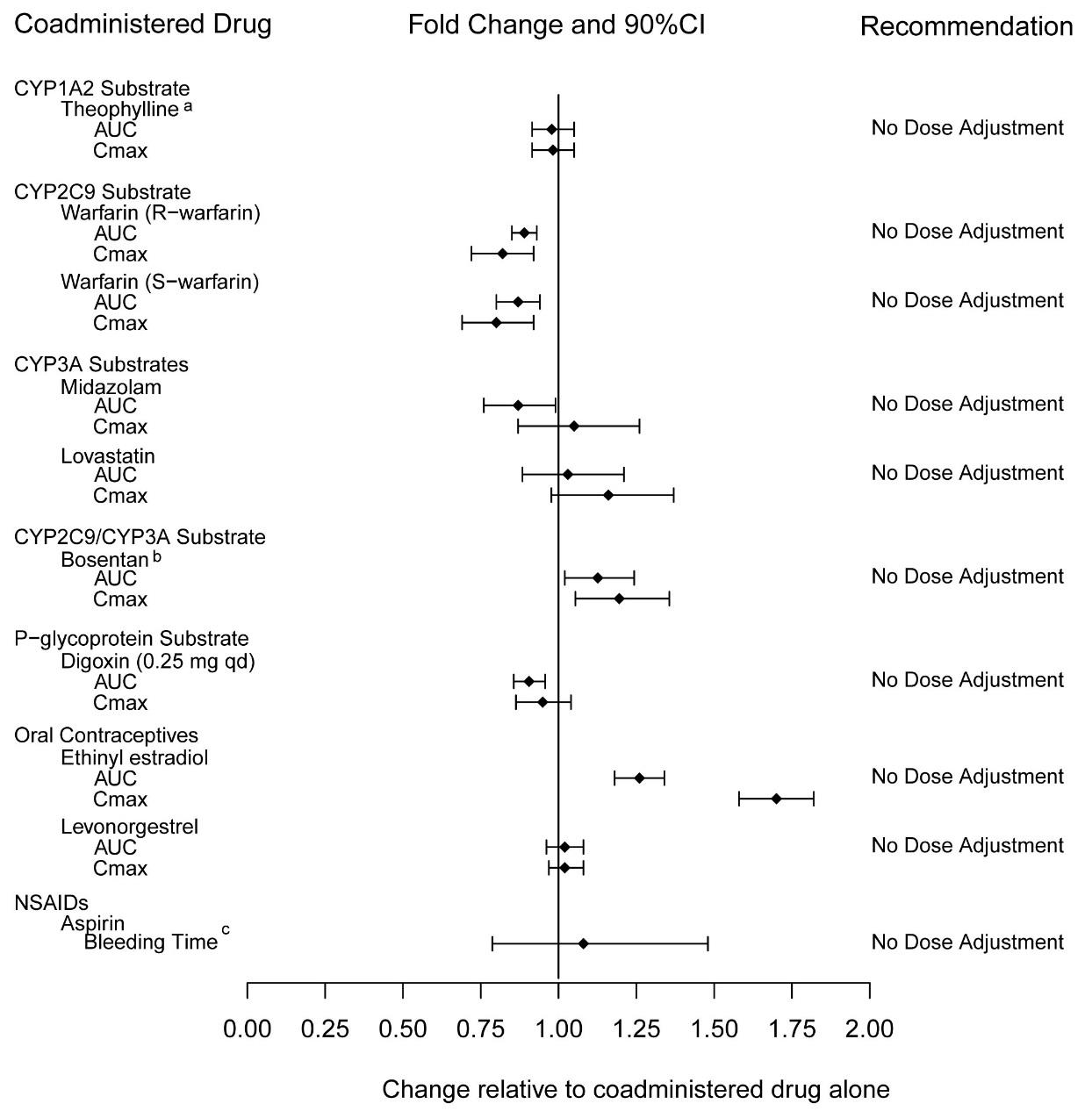
2 DOSAGE AND ADMINISTRATION 40 mg once daily, with or without food. ( 2.1 ) Dividing the dose (40 mg) over the course of the day is not recommended. ( 2.1 ) Use with ritonavir requires dosage adjustments. ( 2.4 ) 2.1 Pulmonary Arterial Hypertension The recommended dose of tadalafil tablets is 40 mg (two 20 mg tablets) taken once daily with or without food. Dividing the dose (40 mg) over the course of the day is not recommended. 2.2 Dose Adjustment in Renal Impairment Mild (creatinine clearance 51 to 80 mL/min) or moderate (creatinine clearance 31 to 50 mL/min): Start dosing at 20 mg once daily. Increase to 40 mg once daily based on individual tolerability. Severe (creatinine clearance <30 mL/min and on hemodialysis): Avoid use of tadalafil tablets because of increased tadalafil exposure (AUC), limited clinical experience, and the lack of ability to influence clearance by dialysis [see Use in Specific Populations ( 8.6 )] . 2.3 Dose Adjustment in Hepatic Impairment Mild or moderate (Child Pugh Class A or B): Because of limited clinical experience in patients with mild to moderate hepatic cirrhosis, consider a starting dose of 20 mg once per day. Severe (Child Pugh Class C): Patients with severe hepatic cirrhosis have not been studied. Avoid use of tadalafil tablets [see Use in Specific Populations ( 8.7 )] . 2.4 Dose Adjustments for Use with Ritonavir Co-administration of Tadalafil Tablets in Patients on Ritonavir In patients receiving ritonavir for at least one week, start tadalafil tablets at 20 mg once daily. Increase to 40 mg once daily based upon individual tolerability [see Drug Interactions ( 7.2 ) and Clinical Pharmacology ( 12.3 )] . Co-administration of Ritonavir in Patients on Tadalafil Tablets Avoid use of tadalafil tablets during the initiation of ritonavir. Stop tadalafil tablets at least 24 hours prior to starting ritonavir. After at least one week following the initiation of ritonavir, resume tadalafil tablets at 20 mg once daily. Increase to 40 mg once daily based upon individual tolerability [see Drug Interactions ( 7.5 ) and Clinical Pharmacology ( 12.3 )] .
