Drug Catalog - Product Detail
TACROLIMUS OINTMENT OINT 0.001 30GM
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 45802-0700-00 | PADAGIS | 30 | 0.1% | OINTMENT |
PACKAGE FILES
Generic Name
TACROLIMUS
Substance Name
TACROLIMUS
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
NDA050777
Description
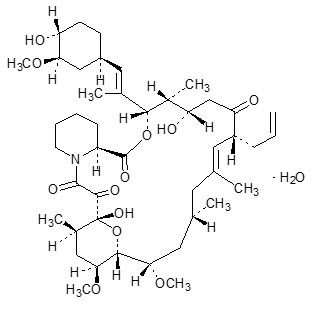
DESCRIPTION Tacrolimus Ointment contains tacrolimus, a macrolide immunosuppressant produced by Streptomyces tsukubaensis . It is for topical dermatologic use only. Chemically, tacrolimus is designated as [3 S -[3 R *[ E (1 S *,3 S *,4 S *)],4 S *,5 R *,8 S *,9 E ,12 R *,14 R *,15 S *,16 R *,18 S *,19 S *,26a R *]]-5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-5,19-dihydroxy-3-[2-(4-hydroxy-3-methoxycyclohexyl)-1-methylethenyl]-14,16-dimethoxy-4,10, 12,18-tetramethyl-8-(2-propenyl)-15,19-epoxy-3H-pyrido[2,1- c ][1,4] oxaazacyclotricosine-1,7,20,21(4H,23H)-tetrone,monohydrate. It has the following structural formula: Tacrolimus has an empirical formula of C 44 H 69 NO 12 ∙H 2 O and a formula weight of 822.03. Each gram of Tacrolimus Ointment contains (w/w) either 0.03% or 0.1% of tacrolimus in a base of mineral oil with all- rac -α-tocopherol, paraffin, propylene carbonate, white petrolatum with butylhydroxytoluene, and white wax. Chemical Structure
How Supplied
HOW SUPPLIED Tacrolimus Ointment 0.03% NDC 45802-390-00 30 gram laminate tube NDC 45802-390-01 60 gram laminate tube NDC 45802-390-02 100 gram laminate tube Tacrolimus Ointment 0.1% NDC 45802-700-00 30 gram laminate tube NDC 45802-700-01 60 gram laminate tube NDC 45802-700-02 100 gram laminate tube Store at room temperature 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F).
Indications & Usage
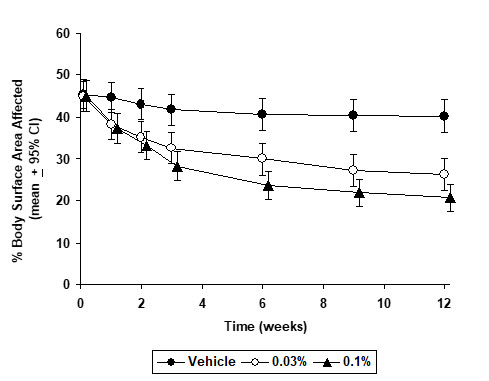
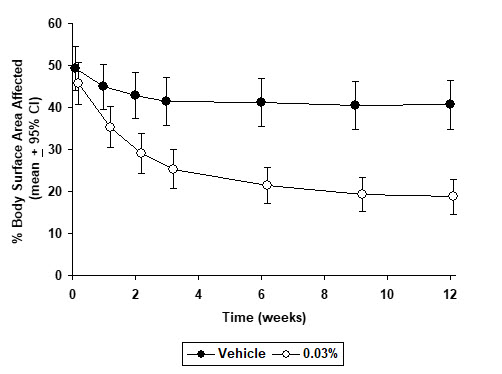
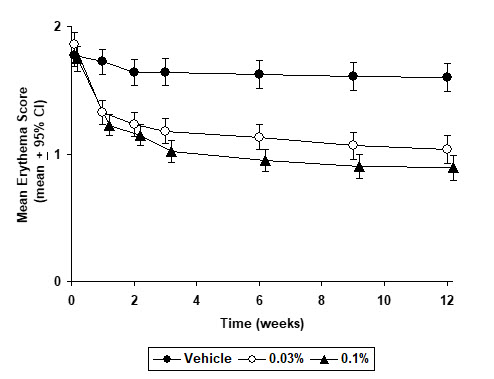
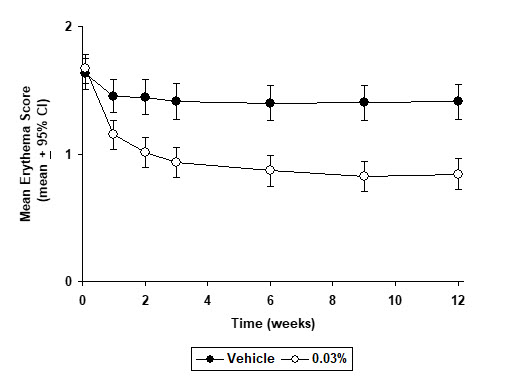
INDICATIONS AND USAGE Tacrolimus Ointment, both 0.03% and 0.1% for adults, and only 0.03% for children aged 2 to 15 years, is indicated as second-line therapy for the short-term and non-continuous chronic treatment of moderate to severe atopic dermatitis in non-immunocompromised adults and children who have failed to respond adequately to other topical prescription treatments for atopic dermatitis, or when those treatments are not advisable. Tacrolimus Ointment is not indicated for children younger than 2 years of age (see boxed WARNING , WARNINGS and PRECAUTIONS: Pediatric Use ).
Dosage and Administration
DOSAGE AND ADMINISTRATION Adult Tacrolimus Ointment 0.03% and 0.1% Apply a thin layer of Tacrolimus Ointment to the affected skin twice daily. The minimum amount should be rubbed in gently and completely to control signs and symptoms of atopic dermatitis. Stop using when signs and symptoms of atopic dermatitis resolve. If signs and symptoms (e.g. itch, rash, and redness) do not improve within 6 weeks, patients should be re-examined by their healthcare provider to confirm the diagnosis of atopic dermatitis. Continuous long-term use of topical calcineurin inhibitors, including Tacrolimus Ointment should be avoided, and application should be limited to areas of involvement with atopic dermatitis. The safety of Tacrolimus Ointment under occlusion, which may promote systemic exposure, has not been evaluated. Tacrolimus Ointment should not be used with occlusive dressings. Pediatric – For Children 2-15 Years Tacrolimus Ointment 0.03% Apply a thin layer of Tacrolimus Ointment, 0.03% to the affected skin twice daily. The minimum amount should be rubbed in gently and completely to control signs and symptoms of atopic dermatitis. Stop using when signs and symptoms of atopic dermatitis resolve. If signs and symptoms (e.g. itch, rash, and redness) do not improve within 6 weeks, patients should be re-examined by their healthcare provider to confirm the diagnosis of atopic dermatitis. Continuous long-term use of topical calcineurin inhibitors, including Tacrolimus Ointment should be avoided, and application should be limited to areas of involvement with atopic dermatitis. The safety of Tacrolimus Ointment under occlusion, which may promote systemic exposure, has not been evaluated. Tacrolimus Ointment should not be used with occlusive dressings.
