Drug Catalog - Product Detail
SUMATRIPTAN SUCCINATE TB 100MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 62756-0522-88 | SUN PHARMACEUTICALS | 100 | 100MG | TABLET |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
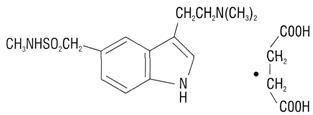
DESCRIPTION Sumatriptan succinate tablets contain sumatriptan (as the succinate), a selective 5-hydroxytryptamine 1 receptor subtype agonist. Sumatriptan succinate is chemically designated as 3-(2-(dimethylamino)ethyl]-N-methyl-indole-5-methanesulfonamide succinate (1:1), and it has the following structure: The molecular formula is C 14 H 21 N 3 O 2 S·C 4 H 6 O 4 representing a molecular weight of 413.5. Sumatriptan succinate is a white to off-white powder that is readily soluble in water and in saline. Each sumatriptan succinate tablet for oral administration contains 35, 70, or 140 mg of sumatriptan succinate, USP equivalent to 25, 50, or 100 mg of sumatriptan, respectively. Each tablet also contains the inactive ingredients colloidal silicon dioxide, croscarmellose sodium, D&C Red # 27 aluminum lake (100 mg only), dibasic calcium phosphate, hypromellose, iron oxide red (100 mg only), magnesium stearate, microcrystalline cellulose, polyethylene glycol (25 & 50 mg only), polysorbate 80 (25 & 50 mg only) propylene glycol (100 mg only), talc, titanium dioxide. chemical-structure
How Supplied
HOW SUPPLIED Sumatriptan succinate tablets, 25, 50, and 100 mg of sumatriptan (base) as the succinate. Sumatriptan succinate tablets, 25 mg are white, triangular-shaped, film-coated tablets debossed with “S” on one side and “I” on the other Bottles of 30 (Child Resistant Cap)………………………..… . NDC 62756-520-83 Bottles of 100 (Child Resistant Cap) ……………………….… NDC 62756-520-88 Bottles of 100 (Non Child Resistant Cap) …………….…….....NDC 62756-520-08 Bottles of 1000 (Non Child Resistant Cap)………..…..……… NDC 62756-520-18 Unit dose blister pack of 9 (1x9) tablets……………….……… NDC 62756-520-69 Unit dose blister pack of 9 (3x3) tablets……………….……… NDC 62756-520-93 Sumatriptan succinate tablets, 50 mg are white, triangular-shaped, film-coated tablets debossed with “S” on one side and “50” on the other Bottles of 30 (Child Resistant Cap)………………….…….…. NDC 62756-521-83 Bottles of 100 (Child Resistant Cap) …………….……..…..…NDC 62756-521-88 Bottles of 100 (Non Child Resistant Cap) …………….……....NDC 62756-521-08 Bottles of 1000 (Non Child Resistant Cap) ………..…..…….. NDC 62756-521-18 Unit dose blister pack of 9 (1x9) tablets ……………….…….. NDC 62756-521-69 Unit dose blister pack of 9 (3x3) tablets……………..….…….NDC 62756-521-93 Sumatriptan succinate tablets, 100 mg are pink, triangular-shaped, film-coated tablets debossed with “S” on one side and “100” on the other. Bottles of 30 (Child Resistant Cap) …………………..……..NDC 62756-522-83 Bottles of 100 (Child Resistant Cap) ………………..………NDC 62756-522-88 Bottles of 100 (Non Child Resistant Cap)…………..…..….. NDC 62756-522-08 Bottles of 1000 (Non Child Resistant Cap) ………..…..……NDC 62756-522-18 Unit dose blister pack of 9 (1x9) tablets……..........................NDC 62756-522-69 Unit dose blister pack of 9 (3x3) tablets..................................NDC 62756-522-93 Store at 20° - 25°C (68° - 77°F); excursions permitted to 15° - 30°C (59° - 86°F) (See USP controlled Room Temperature.)
Indications & Usage
INDICATIONS AND USAGE Sumatriptan succinate tablets are indicated for the acute treatment of migraine attacks with or without aura in adults. Sumatriptan succinate tablets are not intended for the prophylactic therapy of migraine or for use in the management of hemiplegic or basilar migraine (see CONTRAINDICATIONS ). Safety and effectiveness of sumatriptan succinate tablets have not been established for cluster headache, which is present in an older, predominantly male population.
Dosage and Administration
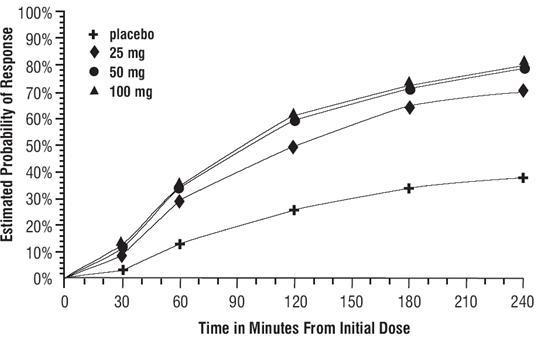
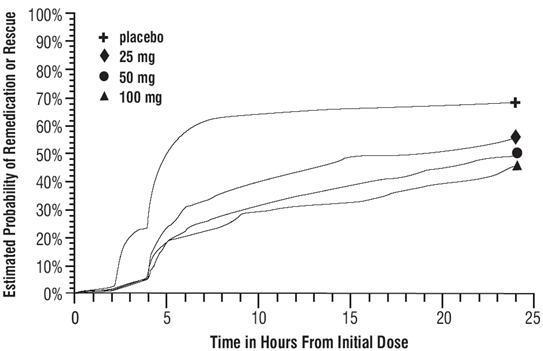
DOSAGE AND ADMINISTRATION In controlled clinical trials, single doses of 25, 50, or 100 mg of sumatriptan succinate tablets were effective for the acute treatment of migraine in adults. There is evidence that doses of 50 and 100 mg may provide a greater effect than 25 mg (see CLINICAL TRIALS ). There is also evidence that doses of 100 mg do not provide a greater effect than 50 mg. Individuals may vary in response to doses of sumatriptan succinate tablets. The choice of dose should therefore be made on an individual basis, weighing the possible benefit of a higher dose with the potential for a greater risk of adverse events. If the headache returns or the patient has a partial response to the initial dose, the dose may be repeated after 2 hours, not to exceed a total daily dose of 200 mg. If a headache returns following an initial treatment with sumatriptan succinate injection, additional single sumatriptan succinate tablets (up to 100 mg/day) may be given with an interval of at least 2 hours between tablet doses. The safety of treating an average of more than 4 headaches in a 30-day period has not been established. Because of the potential of MAO-A inhibitors to cause unpredictable elevations in the bioavailability of oral sumatriptan, their combined use is contraindicated (see CONTRAINDICATIONS ). Hepatic disease/functional impairment may also cause unpredictable elevations in the bioavailability of orally administered sumatriptan. Consequently, if treatment is deemed advisable in the presence of liver disease, the maximum single dose should in general not exceed 50 mg (see CLINICAL PHARMACOLOGY for the basis of this recommendation).
