Drug Catalog - Product Detail
SULINDAC TB 150MG 500
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00591-5661-05 | ACTAVIS PHARMA | 500 | 150MG | TABLET |
PACKAGE FILES


Generic Name
SULINDAC
Substance Name
SULINDAC
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA071795
Description
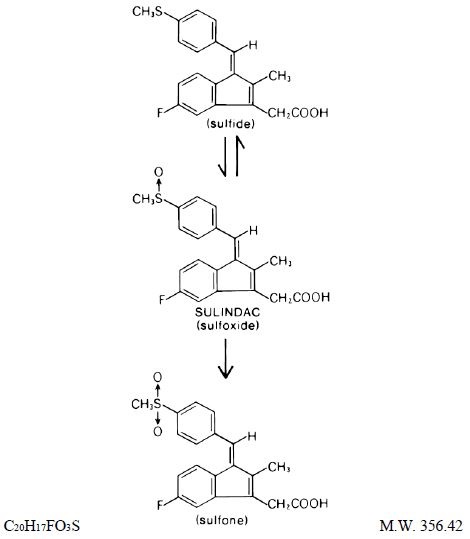
DESCRIPTION Sulindac, USP is a non-steroidal, anti-inflammatory indene derivative designated chemically as (Z)-5-fluoro-2-methyl-1-[[ p -(methylsulfinyl)phenyl]methylene]-1 H -indene-3-acetic acid. It is not a salicylate, pyrazolone or propionic acid derivative. Sulindac, a yellow crystalline compound, is a weak organic acid practically insoluble in water below pH 4.5, but very soluble as the sodium salt or in buffers of pH 6 or higher. Sulindac, USP is available in 150 mg and 200 mg tablets for oral administration. Each tablet contains the following inactive ingredients: magnesium stearate, microcrystalline cellulose, starch (corn) and stearic acid. Following absorption, sulindac undergoes two major biotransformations - reversible reduction to the sulfide metabolite, and irreversible oxidation to the sulfone metabolite. Available evidence indicates that the biological activity resides with the sulfide metabolite. The structural formulas of sulindac, USP and its metabolites are: 1
How Supplied
HOW SUPPLIED Sulindac tablets, USP 150 mg are round, yellow tablets imprinted DAN and 5661 supplied in bottles of 100 (NDC 0591-5661-01) and 500 (NDC 0591-5661-05). Sulindac tablets, USP 200 mg are scored, yellow, round tablets imprinted DAN DAN and 5660 supplied in bottles of 100 (NDC 0591-5660-01) and 500 (NDC 0591-5660-05). Dispense in a well-closed container with child-resistant closure. Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Dispense with Medication Guide available at: www.tevausa.com/medguides Manufactured In India By: Watson Pharma Private Limited Verna, Salcette Goa 403 722 INDIA Manufactured For: Teva Pharmaceuticals Parsippany, NJ 07054 Rev. F 10/2024
Indications & Usage
INDICATIONS AND USAGE Carefully consider the potential benefits and risks of sulindac and other treatment options before deciding to use sulindac. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS ). Sulindac tablets, USP are indicated for acute or long-term use in the relief of signs and symptoms of the following: Osteoarthritis Rheumatoid arthritis** Ankylosing spondylitis Acute painful shoulder (Acute subacromial bursitis/supraspinatus tendinitis) Acute gouty arthritis **The safety and effectiveness of sulindac have not been established in rheumatoid arthritis patients who are designated in the American Rheumatism Association classification as Functional Class IV (incapacitated, largely or wholly bedridden, or confined to wheelchair; little or no self-care).
Dosage and Administration
DOSAGE AND ADMINISTRATION Carefully consider the potential benefits and risks of sulindac and other treatment options before deciding to use sulindac. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS ). After observing the response to initial therapy with sulindac, the dose and frequency should be adjusted to suit an individual patient's needs. Sulindac should be administered orally twice a day with food. The maximum dosage is 400 mg per day. Dosages above 400 mg per day are not recommended. In osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis, the recommended starting dosage is 150 mg twice a day. The dosage may be lowered or raised depending on the response. A prompt response (within one week) can be expected in about one-half of patients with osteoarthritis, ankylosing spondylitis, and rheumatoid arthritis. Others may require longer to respond. In acute painful shoulder (acute subacromial bursitis/supraspinatus tendinitis) and acute gouty arthritis, the recommended dosage is 200 mg twice a day. After a satisfactory response has been achieved, the dosage may be reduced according to the response. In acute painful shoulder, therapy for 7 to 14 days is usually adequate. In acute gouty arthritis, therapy for 7 days is usually adequate.
