Drug Catalog - Product Detail
Spironolactone 100mg Tablet 500
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 53746-0515-05 | AMNEAL PHARMACEUTICALS | 500 | 100MG | TABLET |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
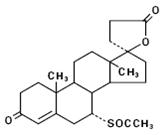
DESCRIPTION Spironolactone oral tablets contain 25 mg, 50 mg, or 100 mg of the aldosterone antagonist spironolactone, USP 17-hydroxy-7α-mercapto-3-oxo-17α-pregn-4-ene-21-carboxylic acid γ- lactone acetate, which has the following structural formula: Spironolactone, USP is practically insoluble in water, soluble in alcohol, and freely soluble in benzene and in chloroform. Inactive ingredients include calcium sulfate, corn starch, croscarmellose sodium, flavor, hypromellose, iron oxide, magnesium stearate, microcrystalline cellulose, polydextrose, polyethylene glycol, polyvinyl alcohol, povidone, talc, titanium dioxide, and triacetin. The 50 mg tablet also contains D&C yellow # 10 and FD&C yellow # 6. Structure
How Supplied
HOW SUPPLIED Spironolactone Tablets, USP 25 mg are white, film-coated, round tablets, debossed “AN” above “511” on one side and plain on other side. They are available as follows: Bottles of 30: NDC 53746-511-30 Bottles of 60: NDC 53746-511-60 Bottles of 90: NDC 53746-511-90 Bottles of 100: NDC 53746-511-01 Bottles of 500: NDC 53746-511-05 Bottles of 1000: NDC 53746-511-10 Spironolactone Tablets, USP 50 mg are yellow, film-coated, round tablets, debossed “AN” bisect “514” on one side and plain on other side. They are available as follows: Bottles of 30: NDC 53746-514-30 Bottles of 60: NDC 53746-514-60 Bottles of 90: NDC 53746-514-90 Bottles of 100: NDC 53746-514-01 Bottles of 500: NDC 53746-514-05 Bottles of 1000: NDC 53746-514-10 Spironolactone Tablets, USP 100 mg are beige, film-coated, round tablets, debossed “AN” on above bisect and “515” below bisect on one side and plain on other side. They are available as follows: Bottles of 30: NDC 53746-515-30 Bottles of 60: NDC 53746-515-60 Bottles of 90: NDC 53746-515-90 Bottles of 100: NDC 53746-515-01 Bottles of 500: NDC 53746-515-05 Bottles of 1000: NDC 53746-515-10 Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Dispense in a tight, light-resistant container. Manufactured by: Amneal Pharmaceuticals Co. (I) Pvt. Ltd. Ahmedabad, INDIA 382220 Distributed by: Amneal Pharmaceuticals Glasgow, KY 42141 Rev. 10-2014-00
Indications & Usage
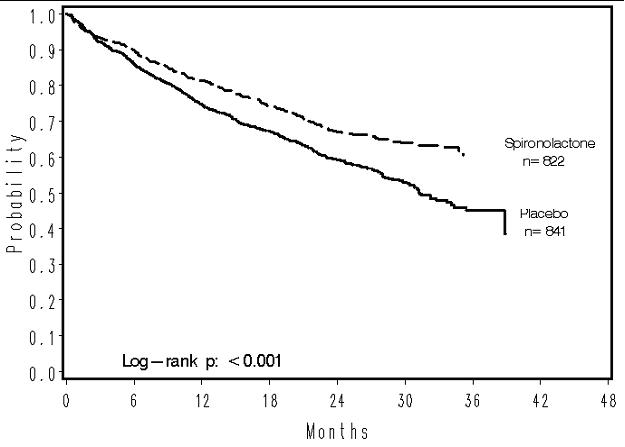
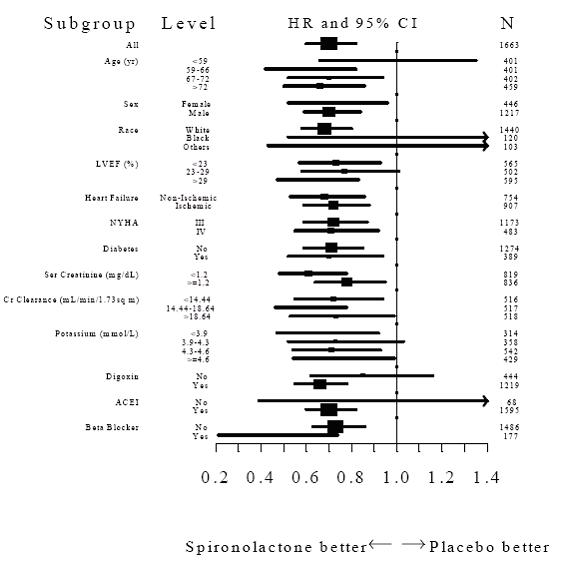
INDICATIONS AND USAGE Spironolactone tablets, USP are indicated in the management of: Primary hyperaldosteronism for: Establishing the diagnosis of primary hyperaldosteronism by therapeutic trial. Short-term preoperative treatment of patients with primary hyperaldosteronism. Long-term maintenance therapy for patients with discrete aldosterone-producing adrenal adenomas who are judged to be poor operative risks or who decline surgery. Long-term maintenance therapy for patients with bilateral micro or macronodular adrenal hyperplasia (idiopathic hyperaldosteronism). Edematous conditions for patients with: Congestive heart failure: For the management of edema and sodium retention when the patient is only partially responsive to, or is intolerant of, other therapeutic measures. Spironolactone tablets, USP are also indicated for patients with congestive heart failure taking digitalis when other therapies are considered inappropriate. Cirrhosis of the liver accompanied by edema and/or ascites: Aldosterone levels may be exceptionally high in this condition. Spironolactone tablets, USP are indicated for maintenance therapy together with bed rest and the restriction of fluid and sodium. Nephrotic syndrome: For nephrotic patients when treatment of the underlying disease, restriction of fluid and sodium intake, and the use of other diuretics do not provide an adequate response. Essential hypertension Spironolactone tablets, USP are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes. Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC). Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly. Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal. Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy. Usually in combination with other drugs, spironolactone tablets, USP are indicated for patients who cannot be treated adequately with other agents or for whom other agents are considered inappropriate. Hypokalemia For the treatment of patients with hypokalemia when other measures are considered inappropriate or inadequate. Spironolactone tablets, USP are also indicated for the prophylaxis of hypokalemia in patients taking digitalis when other measures are considered inadequate or inappropriate. Severe heart failure (NYHA class III – IV) To increase survival, and to reduce the need for hospitalization for heart failure when used in addition to standard therapy. Usage in Pregnancy The routine use of diuretics in an otherwise healthy woman is inappropriate and exposes mother and fetus to unnecessary hazard. Diuretics do not prevent development of toxemia of pregnancy, and there is no satisfactory evidence that they are useful in the treatment of developing toxemia. Edema during pregnancy may arise from pathologic causes or from the physiologic and mechanical consequences of pregnancy. Spironolactone tablets, USP are indicated in pregnancy when edema is due to pathologic causes just as it is in the absence of pregnancy (however, see Precautions: Pregnancy ). Dependent edema in pregnancy, resulting from restriction of venous return by the expanded uterus, is properly treated through elevation of the lower extremities and use of support hose; use of diuretics to lower intravascular volume in this case is unsupported and unnecessary. There is hypervolemia during normal pregnancy which is not harmful to either the fetus or the mother (in the absence of cardiovascular disease), but which is associated with edema, including generalized edema, in the majority of pregnant women. If this edema produces discomfort, increased recumbency will often provide relief. In rare instances, this edema may cause extreme discomfort that is not relieved by rest. In these cases, a short course of diuretics may provide relief and may be appropriate.
Dosage and Administration
DOSAGE AND ADMINISTRATION Primary hyperaldosteronism. Spironolactone tablets, USP may be employed as an initial diagnostic measure to provide presumptive evidence of primary hyperaldosteronism while patients are on normal diets. Long test: Spironolactone tablets, USP are administered at a daily dosage of 400 mg for three to four weeks. Correction of hypokalemia and of hypertension provides presumptive evidence for the diagnosis of primary hyperaldosteronism. Short test: Spironolactone tablets, USP are administered at a daily dosage of 400 mg for four days. If serum potassium increases during spironolactone tablets, USP administration but drops when spironolactone tablets, USP is discontinued, a presumptive diagnosis of primary hyperaldosteronism should be considered. After the diagnosis of hyperaldosteronism has been established by more definitive testing procedures, spironolactone tablets, USP may be administered in doses of 100 to 400 mg daily in preparation for surgery. For patients who are considered unsuitable for surgery, spironolactone tablets, USP may be employed for long-term maintenance therapy at the lowest effective dosage determined for the individual patient. Edema in adults (congestive heart failure, hepatic cirrhosis, or nephrotic syndrome). An initial daily dosage of 100 mg of spironolactone tablets, USP administered in either single or divided doses is recommended, but may range from 25 to 200 mg daily. When given as the sole agent for diuresis, spironolactone tablets, USP should be continued for at least five days at the initial dosage level, after which it may be adjusted to the optimal therapeutic or maintenance level administered in either single or divided daily doses. If, after five days, an adequate diuretic response to spironolactone tablets, USP has not occurred, a second diuretic that acts more proximally in the renal tubule may be added to the regimen. Because of the additive effect of spironolactone tablets, USP when administered concurrently with such diuretics, an enhanced diuresis usually begins on the first day of combined treatment; combined therapy is indicated when more rapid diuresis is desired. The dosage of spironolactone tablets, USP should remain unchanged when other diuretic therapy is added. Essential hypertension. For adults, an initial daily dosage of 50 to 100 mg of spironolactone tablets, USP administered in either single or divided doses is recommended. Spironolactone tablets, USP may also be given with diuretics that act more proximally in the renal tubule or with other antihypertensive agents. Treatment with spironolactone tablets, USP should be continued for at least two weeks, since the maximum response may not occur before this time. Subsequently, dosage should be adjusted according to the response of the patient. Hypokalemia. Spironolactone tablets, USP in a dosage ranging from 25 mg to 100 mg daily is useful in treating a diuretic-induced hypokalemia, when oral potassium supplements or other potassium-sparing regimens are considered inappropriate. Severe heart failure in conjunction with standard therapy (NYHA class III – IV). Treatment should be initiated with spironolactone tablets, USP 25 mg once daily if the patient’s serum potassium is ≤5 mEq/L and the patient’s serum creatinine is ≤ 2.5 mg/dL. Patients who tolerate 25 mg once daily may have their dosage increased to 50 mg once daily as clinically indicated. Patients who do not tolerate 25 mg once daily may have their dosage reduced to 25 mg every other day. SEE WARNINGS: Hyperkalemia in patients with severe heart failure for advice on monitoring serum potassium and serum creatinine.
