Drug Catalog - Product Detail
SOLIFENACIN TAB 10MG 90CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 69097-0261-05 | CIPLA USA | 90 | 10MG | TABLET |
PACKAGE FILES
Generic Name
SOLIFENACINE SUCCINATE
Substance Name
SOLIFENACIN SUCCINATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA209839
Description
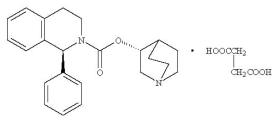
11 DESCRIPTION Solifenacin succinate is a muscarinic receptor antagonist. Chemically, solifenacin succinate is butanedioic acid compound with (1 S )-(3 R )-1-azabicyclo[2.2.2]oct-3-yl 3,4-dihydro-1-phenyl-2(1 H )-iso-quinolinecarboxylate (1:1) having an empirical formula of C 23 H 26 N 2 O 2 •C 4 H 6 O 4 , and a molecular weight of 480.55. The structural formula of solifenacin succinate is: Solifenacin succinate is a white to pale-yellowish-white crystal or crystalline powder. It is freely soluble at room temperature in water, glacial acetic acid, dimethyl sulfoxide, and methanol. Each solifenacin succinate tablet contains 5 or 10 mg of solifenacin succinate and is for oral administration. In addition to the active ingredient solifenacin succinate, each solifenacin succinate tablet also contains the following inactive ingredients: microcystaline cellulose, colloidal silicon dioxide, hypromellose HPMC E-15 and 6 CPS, butylated hydroxyl anisole, magnesium stearate, talc, polyethylene glycol 8000 and titanium dioxide with yellow ferric oxide (5 mg solifenacin succinate tablet) or red ferric oxide (10 mg solifenacin succinate tablet). Image
How Supplied
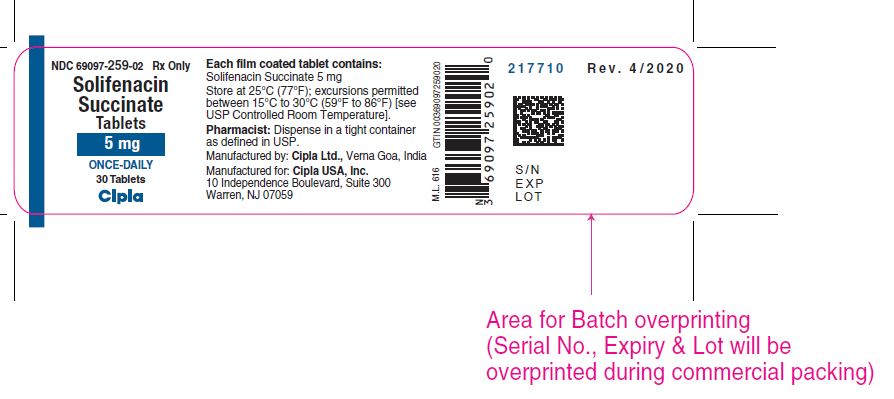
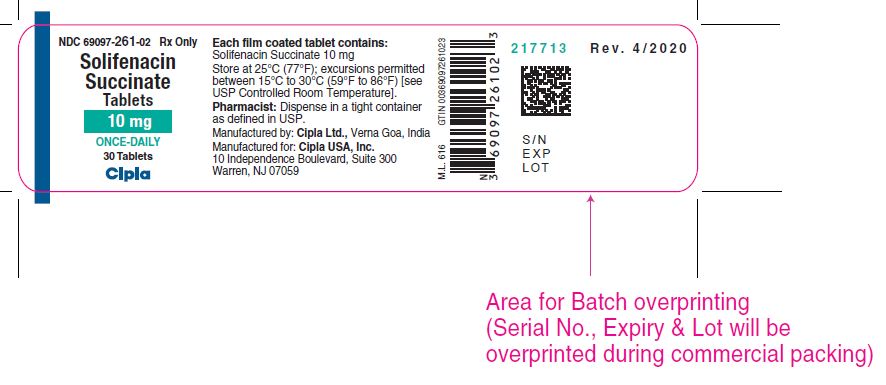
16 HOW SUPPLIED/STORAGE AND HANDLING Solifenacin succinate tablets are supplied as round, film-coated tablets, available in bottles as follows: Each 5 mg tablet is light yellow and debossed with a "'C' and '259' on one side and plain on the other side and is available as follows: Bottle of 30 NDC 69097-259-02 Bottle of 90 NDC 69097-259-05 Bottle of 1000 NDC 69097-259-15 Each 10 mg tablet is light pink and debossed with a 'C' and '261' on one side and plain on the other side and is available as follows: Bottle of 30 NDC 69097-261-02 Bottle of 90 NDC 69097-261-05 Bottle of 1000 NDC 69097-261-15 Store at 25ºC (77ºF) with excursions permitted from 15ºC to 30ºC (59°F to 86ºF) [see USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE Solifenacin succinate is indicated for the treatment of adults with overactive bladder with symptoms of urge urinary incontinence, urgency, and urinary frequency. Solifenacin succinate is a muscarinic antagonist indicated for the treatment of adults with overactive bladder with symptoms of urge urinary incontinence, urgency, and urinary frequency. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION ● 5 mg tablet taken orally once daily, and if well tolerated may be increased to 10 mg once daily. ( 2.1 ) ● Do not exceed the 5 mg dose of solifenacin succinate in patients with: ○ Severe renal impairment creatinine clearance < 30 mL/min/1.73 m 2 . ( 2.2 , 8.6 ) ○ Moderate hepatic impairment (Child-Pugh B). Solifenacin succinate is not recommended in patients with severe hepatic impairment (Child-Pugh C). ( 2.3 , 8.7 ) ○ Concomitant use of strong CYP3A4 inhibitors. ( 2.4 , 7.1 ) 2.1 Dosing Information The recommended oral dose of solifenacin succinate tablets is 5 mg once daily. If the 5 mg dose is well tolerated, the dose may be increased to 10 mg once daily. Solifenacin succinate tablets should be taken with water and swallowed whole. Solifenacin succinate tablets can be administered with or without food 2.2 Dosing Recommendations in Patients with Renal Impairment Do not exceed 5 mg once daily in patients with severe renal impairment (CLcr < 30 mL/min/1.73 m2) [see Use in Specific Populations ( 8.6 )] . 2.3 Dosing Recommendations in Patients with Hepatic Impairment Do not exceed 5 mg once daily in patients with moderate hepatic impairment (Child-Pugh B). Do not use solifenacin succinate tablets in patients with severe hepatic impairment (Child-Pugh C) [see Use in Specific Populations ( 8.7 )] . 2.4 Dosing Recommendations in Patients Taking CYP3A4 Inhibitors Do not exceed 5 mg once daily when solifenacin succinate is administered with strong CYP3A4 inhibitors such as ketoconazole [see Drug Interactions ( 7.1 )] .
