Drug Catalog - Product Detail
SIMAVASTATIN TABS 10MG 1000CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 31722-0511-10 | CAMBER PHARMACEUTICALS | 1000 | 10MG | TABLET |
PACKAGE FILES





Generic Name
SIMVASTATIN
Substance Name
SIMVASTATIN
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA200895
Description
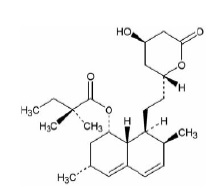
11 DESCRIPTION Simvastatin is a prodrug of 3-hydoroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor that is derived synthetically from a fermentation product of Aspergillus terreus. Simvastatin is butanoic acid, 2,2-dimethyl-,1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-(tetrahydro-4-hydroxy-6-oxo-2 H -pyran-2-yl)-ethyl]-1-naphthalenyl ester, [1 S -[1a,3a,7b,8b( 2S*,4S* ),-8ab]]. The empirical formula of simvastatin is C 25 H 38 O 5 and its molecular weight is 418.57. Its structural formula is: Simvastatin USP is a white to off-white crystalline powder that is practically insoluble in water, freely soluble in chloroform, methanol and alcohol, sparingly soluble in propylene glycol and very slightly soluble in hexane. Simvastatin tablets, USP for oral use contain 5 mg, 10 mg, 20 mg, 40 mg or 80 mg of simvastatin and the following inactive ingredients: ascorbic acid, butylated hydroxyanisole, citric acid monohydrate, hydroxypropyl cellulose, hypromellose, iron oxide yellow, isopropyl alcohol, lactose monohydrate, magnesium stearate, microcrystalline cellulose, pregelatinized starch, talc and titanium dioxide. Additionally the 10 mg, 20 mg, 40 mg and 80 mg strengths contain: iron oxide red. The botanical source for pregelatinized starch is corn starch. simvastatintabsstru
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Simvastatin tablets USP, 5 mg are yellow colored, oval shaped, film coated tablet, debossed with ‘H’ on one side and ‘16’ on other side. They are supplied as follows: Bottles of 30 (NDC 31722-510-30) Bottles of 90 (NDC 31722-510-90) Bottles of 100 (NDC 31722-510-01) Bottles of 1000 (NDC 31722-510-10) Simvastatin tablets USP, 10 mg are pink colored, oval shaped, film coated tablet, debossed with ‘H’ on one side and ‘17’ on other side. They are supplied as follows: Bottles of 30 (NDC 31722-511-30) Bottles of 90 (NDC 31722-511-90) Bottles of 100 (NDC 31722-511-01) Bottles of 1000 (NDC 31722-511-10) Simvastatin tablets USP, 20 mg are brown colored, oval shaped, film coated tablet, debossed with ‘H’ on one side and ‘18’ on other side. They are supplied as follows: Bottles of 30 (NDC 31722-512-30) Bottles of 90 (NDC 31722-512-90) Bottles of 100 (NDC 31722-512-01) Bottles of 1000 (NDC 31722-512-10) Simvastatin tablets USP, 40 mg are brick red colored, oval shaped, film coated tablet, debossed with ‘H’ on one side and ‘19’ on other side. They are supplied as follows: Bottles of 30 (NDC 31722-513-30) Bottles of 90 (NDC 31722-513-90) Bottles of 100 (NDC 31722-513-01) Bottles of 1000 (NDC 31722-513-10) Simvastatin tablets USP, 80 mg are brick red capsule shaped, film coated tablet, debossed with ‘H’ on one side and ‘20’ on other side. They are supplied as follows: Bottles of 30 (NDC 31722-514-30) Bottles of 90 (NDC 31722-514-90) Bottles of 100 (NDC 31722-514-01) Bottles of 500 (NDC 31722-514-05) Storage Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Dispense in a tightly closed container.
Indications & Usage
1 INDICATIONS AND USAGE Simvastatin tablets are indicated: • To reduce the risk of total mortality by reducing risk of coronary heart disease death, non-fatal myocardial infarction and stroke, and the need for coronary and non-coronary revascularization procedures in adults with established coronary heart disease, cerebrovascular disease, peripheral vascular disease, and/or diabetes, who are at high risk of coronary heart disease events. • As an adjunct to diet to reduce low-density lipoprotein cholesterol (LDL-C): o In adults with primary hyperlipidemia. o In adults and pediatric patients aged 10 years and older with heterozygous familial hypercholesterolemia (HeFH). • As an adjunct to other LDL-C-lowering therapies to reduce LDL-C in adults with homozygous familial hypercholesterolemia (HoFH). • As an adjunct to diet for the treatment of adults with: o Primary dysbetalipoproteinemia. o Hypertriglyceridemia. Simvastatin tablets are an HMG-CoA reductase inhibitor indicated: ( 1 ) • To reduce the risk of total mortality by reducing risk of coronary heart disease death, non-fatal myocardial infarction and stroke, and the need for coronary and non-coronary revascularization procedures in adults with established coronary heart disease, cerebrovascular disease, peripheral vascular disease, and/or diabetes, who are at high risk of coronary heart disease events. • As an adjunct to diet to reduce low-density lipoprotein cholesterol (LDL-C): o In adults with primary hyperlipidemia. o In adults and pediatric patients aged 10 years and older with heterozygous familial hypercholesterolemia (HeFH). • As an adjunct to other LDL-C-lowering therapies to reduce LDL-C in adults with homozygous familial hypercholesterolemia (HoFH). • As an adjunct to diet for the treatment of adults with: o Primary dysbetalipoproteinemia. o Hypertriglyceridemia.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION • Important Dosage and Administration Information: ( 2.1 ) o Take simvastatin tablets orally once daily in the evening. o Maximum recommended dosage is simvastatin tablets 40 mg once daily. An 80 mg daily dosage of simvastatin tablets are restricted to patients who have been taking simvastatin 80 mg daily chronically (e.g., for 12 months or more) without evidence of muscle toxicity. o For patients that require a high-intensity statin or are unable to achieve their LDL-C goal receiving simvastatin tablets 40 mg daily, prescribe alternative LDL-C lowering treatment. o Assess LDL-C when clinically appropriate, as early as 4 weeks after initiating, and adjust the dosage if necessary. • Adults: Recommended dosage is 20 mg to 40 mg once daily. ( 2.2 ) • Pediatric Patients Aged 10 Years and Older with HeFH: Recommended dosage is 10 mg to 40 mg once daily. ( 2.3 ) • Patients with Severe Renal Impairment: Recommended starting dosage is simvastatin 5 mg once daily. ( 2.4 , 8.6 ) • See full prescribing information for simvastatin tablets dosage modifications due to drug interactions. ( 2.5 ) 2.1 Important Dosage and Administration Information • Take simvastatin tablets orally once daily in the evening. •The maximum recommended dosage is simvastatin tablets 40 mg once daily [see Dosage and Administration ( 2.2 , 2.3 )] . An 80 mg daily dosage of simvastatintablets are restricted to patients who have been taking simvastatin 80 mg daily chronically (e.g., for 12 months or more) without evidence of muscle toxicity [see Warnings and Precautions ( 5.1 )] . • If as dose is missed, take the missed dose as soon as possible. Do not double the next dose. • For patients that require a high-intensity statin or are unable to achieve their LDL-C goal receiving simvastatin tablets 40 mg daily, prescribe alternative LDL-C-lowering treatment. • Assess LDL-C when clinically appropriate, as early as 4 weeks after initiating simvastatin tablets, and adjust the dosage if necessary. 2.2 Recommended Dosage in Adult Patients The recommended dosage range of simvastatin tablets are 20 mg to 40 mg once daily. 2.3 Recommended Dosage in Pediatric Patients 10 Years of Age and Older with HeFH The recommended dosage range of simvastatin tablets are 10 mg to 40 mg daily. 2.4 Recommended Dosage in Patients with Renal Impairment For patients with severe renal impairment [creatinine clearance (CL cr ) 15 to 29 mL/min], the recommended starting dosage of simvastatin is 5 mg once daily [see Warnings and Precautions (5.1) and Use in Specific Populations (8.6)]. There are no dosage adjustment recommendations for patients with mild or moderate renal impairment. 2.5 Dosage Modifications Due to Drug Interactions Concomitant use of simvastatin tablets with the following drugs requires dosage modification of simvastatin tablets [see Warnings and Precautions ( 5.1 ) and Drug Interactions ( 7.1 )]. Patients taking Lomitapide Reduce the dosage of simvastatin tablets by 50%. Do not exceed simvastatin tablets 20 mg once daily (or 40 mg once daily for patients who have previously taken an 80 mg daily dosage of simvastatin tablets chronically while taking lomitapide) [see Dosage and Administration ( 2.1 )]. Patients taking Verapamil, Diltiazem, or Dronedarone Do not exceed simvastatin tablets 10 mg once daily. Patients taking Amiodarone, Amlodipine, or Ranolazine Do not exceed simvastatin tablets 20 mg once daily.
