Drug Catalog - Product Detail
SERTRALINE TABS 25MG 90CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 16729-0215-15 | ACCORD HEALTHCARE | 90 | 25MG | TABLET |
PACKAGE FILES


Generic Name
SERTRALINE HYDROCHLORIDE
Substance Name
SERTRALINE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA202825
Description
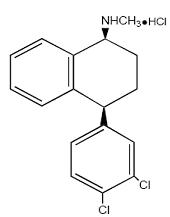
11 DESCRIPTION Sertraline hydrochloride tablets, USP contain sertraline hydrochloride, an SSRI. Sertraline hydrochloride has a molecular weight of 342.7 and has the following chemical name: (1S-cis)-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1-naphthalenamine hydrochloride. The empirical formula C 17 H 17 NCl 2• HCl is represented by the following structural formula: Sertraline hydrochloride is a white crystalline powder that is slightly soluble in water and isopropyl alcohol, and sparingly soluble in ethanol. Sertraline hydrochloride tablets, USP are supplied for oral administration as scored tablets containing sertraline hydrochloride equivalent to 25, 50 and 100 mg of sertraline and the following inactive ingredients: dibasic calcium phosphate anhydrous, D & C Yellow #10 aluminum lake (in 25 mg tablet), FD & C Blue #1 aluminum lake (in 25 mg tablet), FD & C Blue # 2 aluminum lake (in 50 mg tablet), hydroxypropyl cellulose, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, sodium starch glycolate, iron oxide yellow (in 100 mg tablet), and titanium dioxide. Chemical Structure
How Supplied
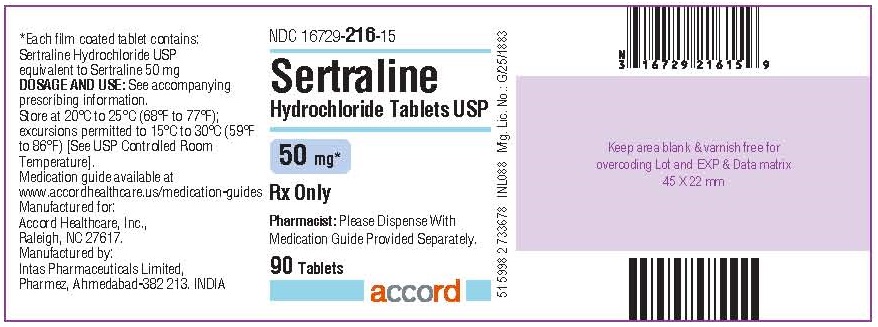
16 HOW SUPPLIED/STORAGE AND HANDLING Sertraline hydrochloride tablets, USP having functional scoring containing sertraline hydrochloride equivalent to 25 mg, 50 mg and 100 mg of sertraline, are supplied as. 25 mg: light green to green colored, round, biconvex, film coated tablets, debossed with “S1” on one side and breakline on other side. NDC 16729-215-10 Bottles of 30’s count with child-resistant closure NDC 16729-215-11 Bottles of 50’s count with child-resistant closure NDC 16729-215-15 Bottles of 90’s count with child-resistant closure NDC 16729-215-43 Unit Dose Packages of 100’s count NDC 16729-215-16 Bottles of 500’s count 50 mg: light blue to blue colored, round, biconvex, film coated tablets, debossed with “S2” on one side and breakline on other side. NDC 16729-216-10 Bottles of 30’s count with child-resistant closure NDC 16729-216-15 Bottles of 90’s count with child-resistant closure NDC 16729-216-01 Bottles of 100’s count with child-resistant closure NDC 16729-216-16 Bottles of 500’s count with child-resistant closure NDC 16729-216-43 Unit Dose Packages of 100’s count 100 mg: yellow colored, round biconvex, film coated tablets, debossed with “S3” on one side and breakline on other side. NDC 16729-217-10 Bottles of 30’s count with child-resistant closure NDC 16729-217-15 Bottles of 90’s count with child-resistant closure NDC 16729-217-01 Bottles of 100’s count with child-resistant closure NDC 16729-217-16 Bottles of 500’s count with child-resistant closure NDC 16729-217-43 Unit Dose Packages of 100’s count Store sertraline hydrochloride at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [See USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE Sertraline hydrochloride tablets are indicated for the treatment of the following [See Clinical Studies (14) ] : Major depressive disorder (MDD) Obsessive-compulsive disorder (OCD) Panic disorder (PD) Posttraumatic stress disorder (PTSD) Social anxiety disorder (SAD) Premenstrual dysphoric disorder (PMDD) Sertraline is a selective serotonin reuptake inhibitor (SSRI) indicated for the treatment of ( 1 ): Major depressive disorder (MDD) Obsessive-compulsive disorder (OCD) Panic disorder (PD) Posttraumatic stress disorder (PTSD) Social anxiety disorder (SAD) Premenstrual dysphoric disorder (PMDD)
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Indication Starting Dosage Maximum Dosage MDD (2.1) 50 mg per day 200 mg per day OCD (2.1) 25 mg per day (ages 6 to 12) 50 mg per day (ages ≥ 13) 200 mg per day PD, PTSD, SAD (2.1) 25 mg per day 200 mg per day PMDD (2.2) continuous dosing 50 mg per day 150 mg per day PMDD (2.2) intermittent dosing 50 mg per day during luteal phase only 100 mg per day during luteal phase only If inadequate response to starting dosage, titrate in 25 mg to 50 mg per day increments once weekly in MDD, OCD, PD, PTSD, and SAD ( 2.1 ) See Full Prescribing Information for titration in PMDD ( 2.2 ) Hepatic impairment: Mild: Recommended starting and maximum dosage is half recommended dosage ( 2.4 ) Moderate or severe: Not recommended ( 2.4 ) When discontinuing sertraline hydrochloride tablets, reduce dose gradually ( 2.6 , 5.4 ) 2.1 Dosage in Patients with MDD, OCD, PD, PTSD, and SAD The recommended initial dosage and maximum sertraline hydrochloride tablets dosage in patients with MDD, OCD, PD, PTSD, and SAD are displayed in Table 1 below. A dosage of 25 mg or 50 mg per day is the initial therapeutic dosage. For adults and pediatric patients, subsequent dosages may be increased in case of an inadequate response in 25 mg to 50 mg per day increments once a week, depending on tolerability, up to a maximum of 200 mg per day. Given the 24-hour elimination half-life of sertraline hydrochloride, the recommended interval between dose changes is one week. Table 1: Recommended Daily Dosage of Sertraline Hydrochloride Tablets in Patients with MDD, OCD, PD, PTSD, and SAD Indication Starting Dose Therapeutic Range Adults MDD 50 mg 50 mg to 200 mg OCD 50 mg PD, PTSD, SAD 25 mg Pediatric Patients OCD (ages 6 to 12 years old) 25 mg 50 mg to 200 mg OCD (ages 13 to 17 years old) 50 mg 2.2 Dosage in Patients with PMDD The recommended starting sertraline hydrochloride tablets dosage in adult women with PMDD is 50 mg per day. Sertraline hydrochloride tablets may be administered either continuously (every day throughout the menstrual cycle) or intermittently (only during the luteal phase of the menstrual cycle, i.e., starting the daily dosage 14 days prior to the anticipated onset of menstruation and continuing through the onset of menses). Intermittent dosing would be repeated with each new cycle. When dosing continuously, patients not responding to a 50 mg dosage may benefit from dosage increases at 50 mg increments per menstrual cycle up to 150 mg per day. When dosing intermittently, patients not responding to a 50 mg dosage may benefit from increasing the dosage up to a maximum of 100 mg per day during the next menstrual cycle (and subsequent cycles) as follows: 50 mg per day during the first 3 days of dosing followed by 100 mg per day during the remaining days in the dosing cycle. 2.3 Screen for Bipolar Disorder Prior to Starting Sertraline Hydrochloride Tablets Prior to initiating treatment with sertraline hydrochloride tablets or another antidepressant, screen patients for a personal or family history of bipolar disorder, mania, or hypomania [See Warnings and Precautions (5.4) ]. 2.4 Dosage Modifications in Patients with Hepatic Impairment Both the recommended starting dosage and therapeutic range in patients with mild hepatic impairment (Child Pugh scores 5 or 6) are half the recommended daily dosage [See Dosage and Administration (2.1 , 2.2) ]. The use of sertraline hydrochloride tablets in patients with moderate (Child Pugh scores 7 to 9) or severe hepatic impairment (Child Pugh scores 10 to 15) is not recommended [See Use in Specific Populations (8.6) , Clinical Pharmacology (12.3) ]. 2.5 Switching Patients to or from a Monoamine Oxidase Inhibitor Antidepressant At least 14 days must elapse between discontinuation of a monoamine oxidase inhibitor (MAOI) antidepressant and initiation of sertraline hydrochloride tablets. In addition, at least 14 days must elapse after stopping sertraline hydrochloride tablets before starting an MAOI antidepressant [See Contraindications (4) , Warnings and Precautions (5.2) ]. 2.6 Discontinuation of Treatment with Sertraline Hydrochloride Tablets Adverse reactions may occur upon discontinuation of sertraline hydrochloride tablets [See Warnings and Precautions (5.5) ]. Gradually reduce the dosage rather than stopping sertraline hydrochloride tablets abruptly whenever possible.
