Drug Catalog - Product Detail
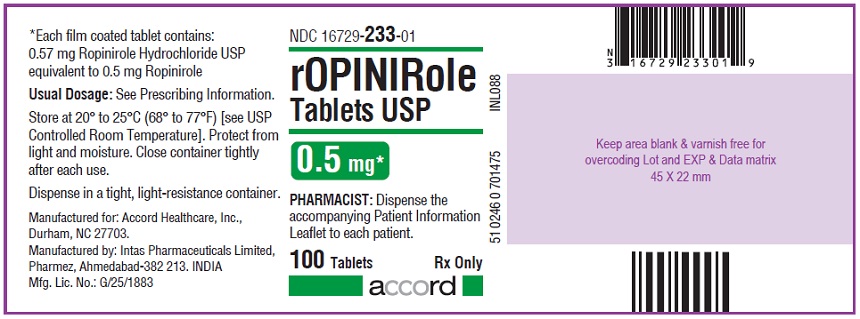
ROPINIROLE TABS 0.5MG 100CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 16729-0233-01 | ACCORD HEALTHCARE | 100 | 0.5MG | TABLET |
PACKAGE FILES
Generic Name
ROPINIROLE
Substance Name
ROPINIROLE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA204022
Description
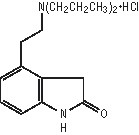
11 DESCRIPTION Ropinirole tablets, USP contains ropinirole, a non-ergoline dopamine agonist, as the hydrochloride salt. The chemical name of ropinirole hydrochloride is 4-[2-(dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-one and the molecular formula is C 16 H 24 N 2 O•HCl. The molecular weight is 296.84 (260.38 as the free base). The structural formula is: Ropinirole hydrochloride is a white to yellow solid with a melting range of 243°C to 250°C and a solubility of 133 mg/mL in water. Each film-coated tablet contains ropinirole hydrochloride equivalent to ropinirole, 0.25, 0.5, 1, 2, 3, 4, or 5 mg. Inactive ingredients consist of: croscarmellose sodium, lactose monohydrate, and magnesium stearate, microcrystalline cellulose. The film coating of the tablet consists of following inactive ingredients: 0.25 mg: hypromellose, polyethylene glycol, polysorbate 80 and titanium dioxide. 0.5 mg: FD&C Blue #2/Indigo carmine aluminum lake, hypromellose, iron oxide red, iron oxide yellow, polyethylene glycol and titanium dioxide. 1 mg: FD&C Blue #2/Indigo carmine aluminum lake, hypromellose, iron oxide yellow, polyethylene glycol and titanium dioxide. 2 mg: hypromellose, iron oxide red, iron oxide yellow, polyethylene glycol and titanium dioxide. 3 mg: carmine, FD&C Blue #2/Indigo carmine aluminum lake, FD&C Yellow No. 6/Sunset yellow FCF aluminum lake, hypromellose, polyethylene glycol and titanium dioxide. 4 mg: hypromellose, iron oxide black, iron oxide red, iron oxide yellow, polyethylene glycol and titanium dioxide. 5 mg: FD&C Blue #2/Indigo carmine aluminum lake, hypromellose, polyethylene glycol, polysorbate 80 and titanium dioxide. chemical structure
How Supplied
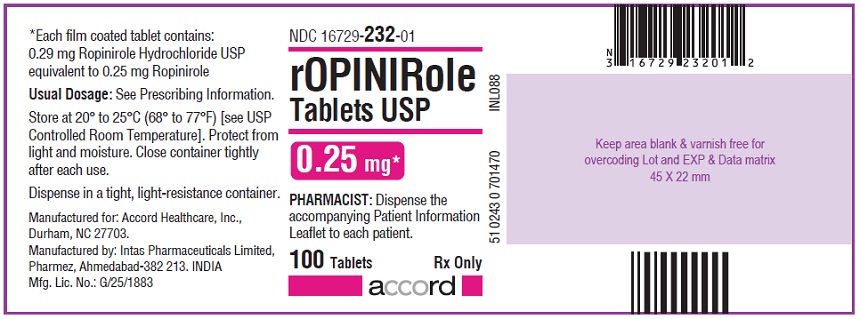
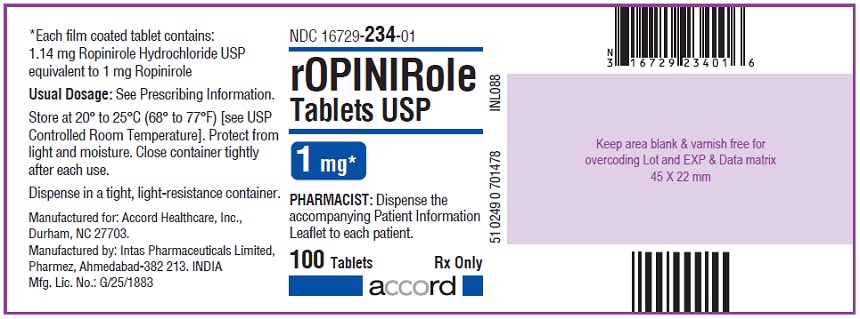
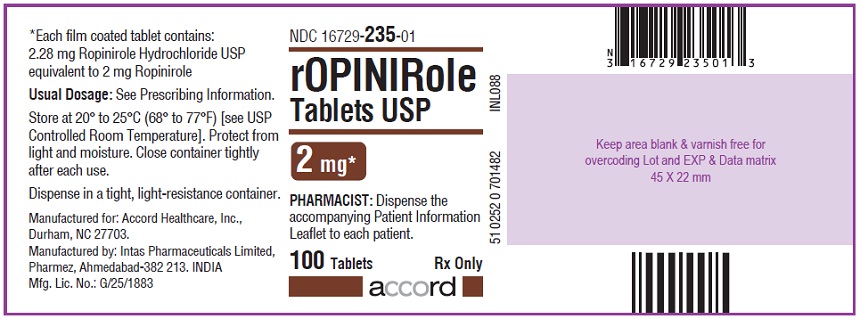
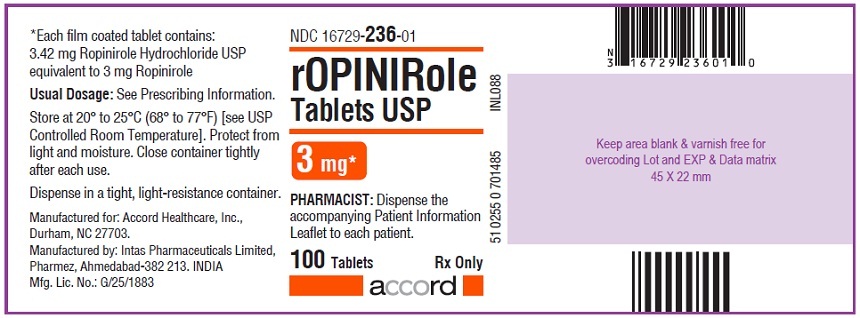
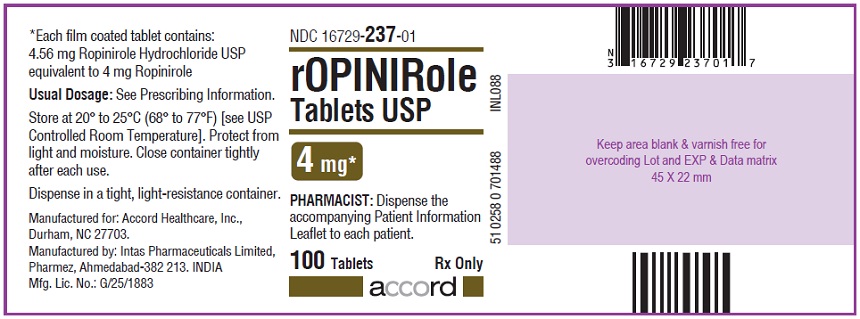
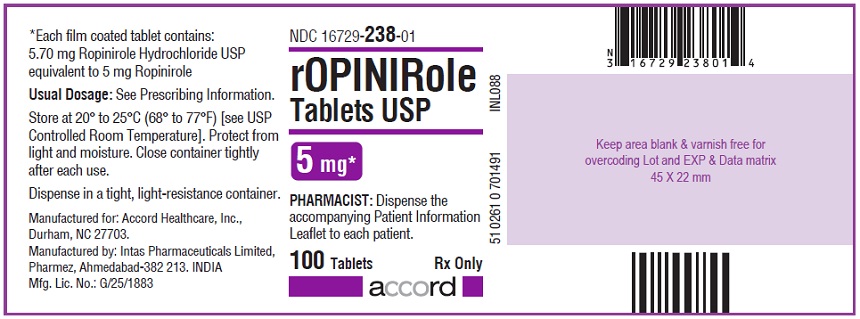
16 HOW SUPPLIED/STORAGE AND HANDLING Ropinirole tablets, USP are available as follows: 0.25 mg: White to off white, round, biconvex, film coated tablets debossed with "R6" on one side and plain on other side. Bottles of 100’s count (NDC 16729-232-01) comes with a child-resistant closure. 0.5 mg: Yellow, round, biconvex, film coated tablets debossed with "R7" on one side and plain on other side. Bottles of 100’s count (NDC 16729-233-01) comes with a child-resistant closure. 1 mg: Green, round, biconvex, film coated tablets debossed with "R1" on one side and plain on other side. Bottles of 100’s count (NDC 16729-234-01) comes with a child-resistant closure. 2 mg: Pink, round, biconvex, film coated tablets debossed with "R2" on one side and plain on other side. Bottles of 100’s count (NDC 16729-235-01) comes with a child-resistant closure. 3 mg: Purple to light purple, round, biconvex, film coated tablets debossed with "R3" on one side and plain on other side. Bottles of 100’s count (NDC 16729-236-01) comes with a child-resistant closure. 4 mg: Brown to pale brown, round, biconvex, film coated tablets debossed with "R4" on one side and plain on other side. Bottles of 100’s count (NDC 16729-237-01) comes with a child-resistant closure. 5 mg: Blue, round, biconvex, film coated tablets debossed with "R5" on one side and plain on other side. Bottles of 100’s count (NDC 16729-238-01) comes with a child-resistant closure. Storage Store at room temperature between 20°C and 25°C (68°F and 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Protect from light and moisture. Close container tightly after each use.
Indications & Usage
1 INDICATIONS AND USAGE Ropinirole tablets are a non-ergoline dopamine agonist indicated for the treatment of Parkinson’s disease (PD) and moderate-to-severe primary Restless Legs Syndrome (RLS). ( 1.1, 1.2 ) 1.1 Parkinson’s Disease Ropinirole tablets are indicated for the treatment of Parkinson’s disease. 1.2 Restless Legs Syndrome Ropinirole tablets are indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS).
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Ropinirole tablets can be taken with or without food. ( 2.1 ) Retitration of ropinirole hydrochloride may be warranted if therapy is interrupted. ( 2.1 ) Parkinson’s Disease: The recommended starting dose is 0.25 mg taken three times daily; titrate to a maximum daily dose of 24 mg. (2.2) Renal Impairment: The maximum recommended dose is 18 mg/day in patients with end-stage renal disease on hemodialysis. (2.2) Restless Legs Syndrome : The recommended starting dose is 0.25 mg once daily, 1 to 3 hours before bedtime, titrate to a maximum recommended dose of 4 mg daily. (2.3) Renal Impairment: The maximum recommended dose is 3 mg/day in patients with end-stage renal disease on hemodialysis. (2.3) 2.1 General Dosing Recommendations Ropinirole tablets can be taken with or without food [see Clinical Pharmacology (12.3) ] . If a significant interruption in therapy with ropinirole hydrochloride has occurred, retitration of therapy may be warranted. 2.2 Dosing for Parkinson’s Disease The recommended starting dose of ropinirole hydrochloride for Parkinson’s disease is 0.25 mg 3 times daily. Based on individual patient therapeutic response and tolerability, if necessary, the dose should then be titrated with weekly increments as described in Table 1. After Week 4, if necessary, the daily dose may be increased by 1.5 mg/day on a weekly basis up to a dose of 9 mg/day, and then by up to 3 mg/day weekly up to a maximum recommended total daily dose of 24 mg/day (8 mg 3 times daily). Doses greater than 24 mg/day have not been tested in clinical trials. Table 1. Ascending-Dose Schedule of Ropinirole Hydrochloride for Parkinson’s Disease Week Dosage Total Daily Dose 1 0.25 mg 3 times daily 0.75 mg 2 0.5 mg 3 times daily 1.5 mg 3 0.75 mg 3 times daily 2.25 mg 4 1 mg 3 times daily 3 mg Ropinirole tablets should be discontinued gradually over a 7-day period in patients with Parkinson’s disease [ see Warnings and Precautions (5.8) ] . The frequency of administration should be reduced from 3 times daily to twice daily for 4 days. For the remaining 3 days, the frequency should be reduced to once daily prior to complete withdrawal of ropinirole tablets. Renal Impairment No dose adjustment is necessary in patients with moderate renal impairment (creatinine clearance of 30 to 50 mL/min). The recommended initial dose of ropinirole for patients with end-stage renal disease on hemodialysis is 0.25 mg 3 times a day. Further dose escalations should be based on tolerability and need for efficacy. The recommended maximum total daily dose is 18 mg/day in patients receiving regular dialysis. Supplemental doses after dialysis are not required. The use of ropinirole hydrochloride in patients with severe renal impairment without regular dialysis has not been studied. 2.3 Dosing for Restless Legs Syndrome The recommended adult starting dose for RLS is 0.25 mg once daily 1 to 3 hours before bedtime. After 2 days, if necessary, the dose can be increased to 0.5 mg once daily, and to 1 mg once daily at the end of the first week of dosing, then as shown in Table 2 as needed to achieve efficacy. Titration should be based on individual patient therapeutic response and tolerability, up to a maximum recommended dose of 4 mg daily. For RLS, the safety and effectiveness of doses greater than 4 mg once daily have not been established. Table 2. Dose Titration Schedule of Ropinirole Hydrochloride for Restless Legs Syndrome Day/Week Dose to be taken once daily 1 to 3 hours before bedtime Days 1 and 2 0.25 mg Days 3 to 7 0.5 mg Week 2 1 mg Week 3 1.5 mg Week 4 2 mg Week 5 2.5 mg Week 6 3 mg Week 7 4 mg When discontinuing ropinirole hydrochloride in patients with RLS, gradual reduction of the daily dose is recommended [see Warnings and Precautions (5.8, 5.9) ] . Renal Impairment No dose adjustment is necessary in patients with moderate renal impairment (creatinine clearance of 30 to 50 mL/min). The recommended initial dose of ropinirole for patients with end-stage renal disease on hemodialysis is 0.25 mg once daily. Further dose escalations should be based on tolerability and need for efficacy. The recommended maximum total daily dose is 3 mg/day in patients receiving regular dialysis. Supplemental doses after dialysis are not required. The use of ropinirole hydrochloride in patients with severe renal impairment without regular dialysis has not been studied.
