Drug Catalog - Product Detail
ROPINIROLE 2MG ER TABLETS 90
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00228-3658-09 | ACTAVIS PHARMA | 90 | 2MG | TABLET |
PACKAGE FILES





Generic Name
ROPINIROLE
Substance Name
ROPINIROLE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA090869
Description
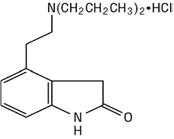
11 DESCRIPTION Ropinirole Extended-Release Tablets, USP contain ropinirole, a non-ergoline dopamine agonist as the hydrochloride salt. The chemical name of ropinirole hydrochloride, USP is 4-[2-(dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-one and has a molecular formula of C 16 H 24 N 2 O•HCl. The molecular weight is 296.84 (260.38 as the free base). The structural formula is: Ropinirole hydrochloride, USP is a white to yellow solid with a melting range of 243º to 250ºC and a solubility of 133 mg/mL in water. Each Ropinirole Extended-Release Tablet, USP for oral administration is oval-shaped and contains 2.28 mg, 4.56 mg, 6.84 mg, 9.12 mg, or 13.68 mg of ropinirole hydrochloride, USP equivalent to ropinirole 2 mg, 4 mg, 6 mg, 8 mg, or 12 mg, respectively. Inactive ingredients consist of colloidal silicon dioxide, croscarmellose sodium, hydrogenated castor oil, hypromellose, lactose monohydrate, magnesium stearate, maltodextrin, and titanium dioxide. In addition the 2 mg tablet also contains iron oxide black, iron oxide red, iron oxide yellow, polyethylene glycol, polyvinyl-alcohol, and talc. The 4 mg tablet also contains black iron oxide, FD&C Blue #2/Indigo Carmine Aluminum Lake, FD&C Yellow #6/Sunset Yellow FCF Aluminum Lake, polydextrose, polyethylene glycol, and triacetin. The 6 mg tablet also contains polyethylene glycol, polyvinyl-alcohol, and talc. The 8 mg tablet also contains iron oxide red, polyethylene glycol, polyvinyl-alcohol, and talc. The 12 mg tablet also contains iron oxide yellow, polyethylene glycol, polyvinyl-alcohol, and talc. This drug product conforms to USP Dissolution Test 3. 54887e95-figure-06
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Each oval-shaped tablet contains ropinirole hydrochloride, USP equivalent to the labeled amount of ropinirole as follows: 2 mg, pink, oval-shaped, unscored, film-coated tablets, debossed with and 658 on one side and plain on the other side, in bottles of 30 (NDC 0228-3658-03) and 90 (NDC 0228-3658-09). 4 mg, blue, oval-shaped, unscored, film-coated tablets, debossed with and 659 on one side and plain on the other side, in bottles of 30 (NDC 0228-3659-03) and 90 (NDC 0228-3659-09). 6 mg, white to off-white, oval-shaped, unscored, film-coated tablets, debossed with and 640 on one side and plain on the other side, in bottles of 30 (NDC 0228-3640-03). 8 mg, red, oval-shaped, unscored, film-coated tablets, debossed with and 660 on one side and plain on the other side, in bottles of 30 (NDC 0228-3660-03) and 90 (NDC 0228-3660-09). 12 mg, yellow, oval-shaped, unscored, film-coated tablets, debossed with and 661 on one side and plain on the other side, in bottles of 30 (NDC 0228-3661-03). Storage Store at 25ºC (77ºF); excursions permitted to 15º to 30ºC (59º to 86ºF) [See USP Controlled Room Temperature]. Dispense in a tight, light-resistant container as defined in the USP. 54887e95-figure-07 54887e95-figure-08 54887e95-figure-09 54887e95-figure-10 54887e95-figure-11
Indications & Usage
1 INDICATIONS AND USAGE Ropinirole extended-release tablets are indicated for the treatment of Parkinson’s disease. Ropinirole extended-release tablets are a non-ergoline dopamine agonist indicated for the treatment of Parkinson’s disease. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Ropinirole extended-release tablets are taken once daily, with or without food; tablets must be swallowed whole and not be chewed, crushed, or divided. ( 2.1 ) The recommended starting dose is 2 mg taken once daily for 1 to 2 weeks; the dose should be increased by 2 mg/day at 1 week or longer intervals. The maximum recommended dose of ropinirole extended-release tablets is 24 mg/day. ( 2.2 , 14.2 ) Renal Impairment: In patients with end-stage renal disease on hemodialysis, the maximum recommended dose is 18 mg/day. ( 2.2 ) If ropinirole extended-release tablets must be discontinued, it should be tapered gradually over a 7-day period; retitration of ropinirole extended-release tablets may be warranted if therapy is interrupted. ( 2.1 , 2.2 ) Patients may be switched directly from immediate-release ropinirole to ropinirole extended-release tablets; the initial switching dose of ropinirole extended-release tablets should approximately match the total daily dose of immediate-release ropinirole. ( 2.3 ) 2.1 General Dosing Recommendations Ropinirole extended-release tablets are taken once daily, with or without food [see Clinical Pharmacology (12.3) ]. Tablets must be swallowed whole and must not be chewed, crushed, or divided. If a significant interruption in therapy with ropinirole extended-release tablets has occurred, retitration of therapy may be warranted. 2.2 Dosing for Parkinson’s Disease The recommended starting dose of ropinirole extended-release tablets is 2 mg taken once daily for 1 to 2 weeks, followed by increases of 2 mg/day at weekly or longer intervals, based on therapeutic response and tolerability. Monitor patients at least weekly during dose titration. Too rapid a rate of titration may lead to the selection of a dose that does not provide additional benefit, but increases the risk of adverse reactions. In fixed-dose studies designed to characterize the dose response to ropinirole extended-release tablets, there was no additional therapeutic benefit shown in patients with advanced stage Parkinson’s disease taking daily doses greater than 8 mg/day, or with early stage Parkinson’s disease taking doses greater than 12 mg/day [see Clinical Studies ( 14.1 and 14.2 )] . Although the maximum recommended dose of ropinirole extended-release tablets is 24 mg, patients with advanced Parkinson’s disease should generally be maintained at daily doses of 8 mg or lower and patients with early Parkinson’s disease should generally be maintained at daily doses 12 mg or lower. Ropinirole extended-release tablets should be discontinued gradually over a 7-day period [see Warnings and Precautions ( 5.9 )] . Renal Impairment No dose adjustment is necessary in patients with moderate renal impairment (creatinine clearance of 30 to 50 mL/min). The recommended initial dose of ropinirole extended-release tablets for patients with end-stage renal disease on hemodialysis is 2 mg once daily. Further dose escalations should be based on tolerability and need for efficacy. The recommended maximum total daily dose is 18 mg/day in patients receiving regular dialysis. Supplemental doses after dialysis are not required. The use of ropinirole extended-release tablets in patients with severe renal impairment without regular dialysis has not been studied. 2.3 Switching from Immediate-Release Ropinirole Tablets to Ropinirole Extended-Release Tablets Patients may be switched directly from immediate-release ropinirole tablets to ropinirole extended-release tablets. The initial dose of ropinirole extended-release tablets should approximately match the total daily dose of the immediate-release formulation of ropinirole, as shown in Table 1. Table 1. Conversion from Immediate-Release Ropinirole Tablets to Ropinirole Extended-Release Tablets Immediate-Release Ropinirole Tablets Ropinirole Extended-Release Tablets Total Daily Dose (mg) Total Daily Dose (mg) 0.75 to 2.25 2 3 to 4.5 4 6 6 7.5 to 9 8 12 12 15 16 18 18 21 20 24 24 Following conversion to ropinirole extended-release tablets, the dose may be adjusted depending on therapeutic response and tolerability [see Dosage and Administration (2.2) ]. 2.4 Effect of Gastrointestinal Transit Time on Medication Release Ropinirole extended-release tablets are designed to release medication over a 24-hour period. If rapid gastrointestinal transit occurs, there may be risk of incomplete release of medication and medication residue being passed in the stool.
