Drug Catalog - Product Detail
RIVASTIGMNE TRNSDRML SYS 4.6MG PTCH 30CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65162-0825-34 | AMNEAL PHARMACEUTICALS | 30 | 4.6MG/24HR | TRANSDERMAL SYSTEM |
PACKAGE FILES



Generic Name
RIVASTIGMINE
Substance Name
RIVASTIGMINE
Product Type
HUMAN PRESCRIPTION DRUG
Route
TRANSDERMAL
Application Number
ANDA207308
Description
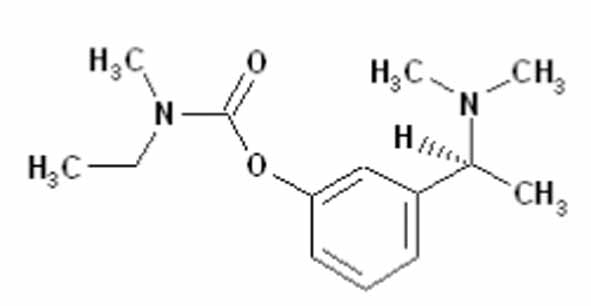
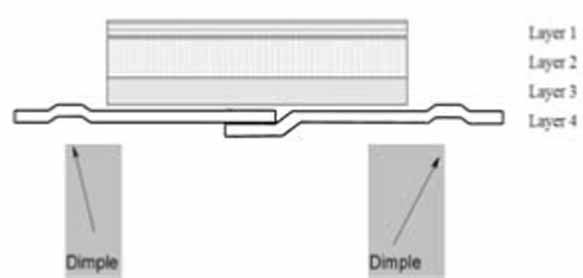
11 DESCRIPTION Rivastigmine transdermal system contains rivastigmine, USP, a reversible cholinesterase inhibitor known chemically as (S)- 3-[1-(dimethylamino) ethyl]phenyl ethylmethylcarbamate. It has an empirical formula of C 14 H 22 N 2 O 2 as the base and a molecular weight of 250.34 g/mol (as the base). Rivastigmine, USP is a viscous, clear, and colorless to yellow to very slightly brown liquid that is sparingly soluble in water and very soluble in ethanol, acetonitrile, n-octanol and ethyl acetate. The distribution coefficient at 37°C in n-octanol/phosphate buffer solution pH 7 is 4.27. Rivastigmine transdermal system is a patch for transdermal administration. The patch is a 4-layer laminate containing the backing layer, drug matrix, adhesive matrix and overlapping release liner (see Figure 1). The release liner is removed and discarded prior to use. Figure 1: Cross Section of the Rivastigmine Transdermal System Layer 1: Backing Film Layer 2: Drug Product (Acrylic) Matrix Layer 3: Adhesive (Silicone) Matrix Layer 4: Release Liner (removed at time of use) Excipients within the formulation include acrylic copolymers, dimethicone, silicone adhesive applied to a pigmented polyethylene polyester backing, and vitamin E. chem structure figure 1
How Supplied
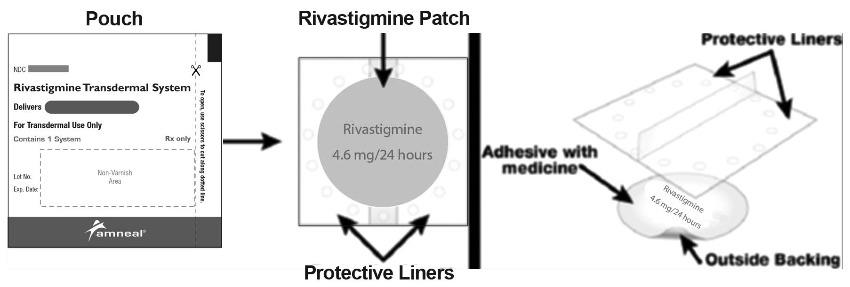
16 HOW SUPPLIED/STORAGE AND HANDLING Rivastigmine Transdermal System: 4.6 mg/24 hours A 5 cm 2 circular patch, consisting of transparent to translucent adhesive layer on light pink colored backing film printed with “Rivastigmine 4.6 mg/24 hours” in red ink, protected by a removable, transparent, overlapping release liner with dimples surrounding the perimeter of the patch. Each patch of 5 cm 2 contains 9 mg rivastigmine base with in vivo release rate of 4.6 mg/24 hours. They are available as follows: Carton of 30 NDC 65162-825-34 Rivastigmine Transdermal System: 9.5 mg/24 hours A 10 cm 2 circular patch, consisting of transparent to translucent adhesive layer on light pink colored backing film printed with “Rivastigmine 9.5 mg/24 hours” in red ink, protected by a removable, transparent, overlapping release liner with dimples surrounding the perimeter of the patch. Each patch of 10 cm 2 contains 18 mg rivastigmine base with in vivo release rate of 9.5 mg/24 hours. They are available as follows: Carton of 30 NDC 65162-826-34 Rivastigmine Transdermal System: 13.3 mg/24 hours A 15 cm 2 circular patch, consisting of transparent to translucent adhesive layer on light pink colored backing film printed with “Rivastigmine 13.3 mg/24 hours” in red ink, protected by a removable, transparent, overlapping release liner with dimples surrounding the perimeter of the patch. Each patch of 15 cm 2 contains 27 mg rivastigmine base with in vivo release rate of 13.3 mg/24 hours. Carton of 30 NDC 65162-749-34 Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Keep rivastigmine transdermal system in the individual sealed pouch until use. Each pouch contains 1 patch. Used systems should be folded, with the adhesive surfaces pressed together, and discarded safely.
Indications & Usage
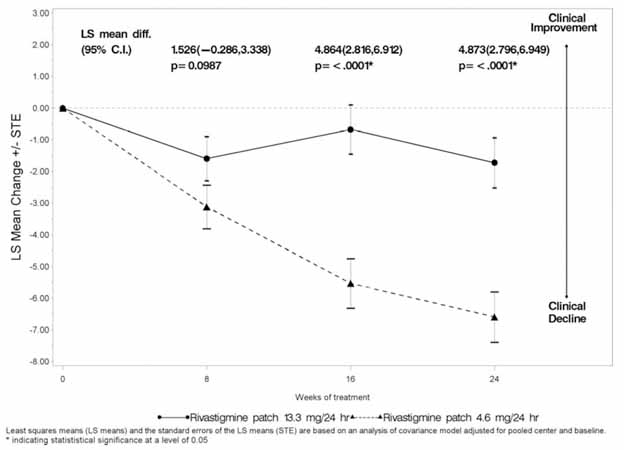
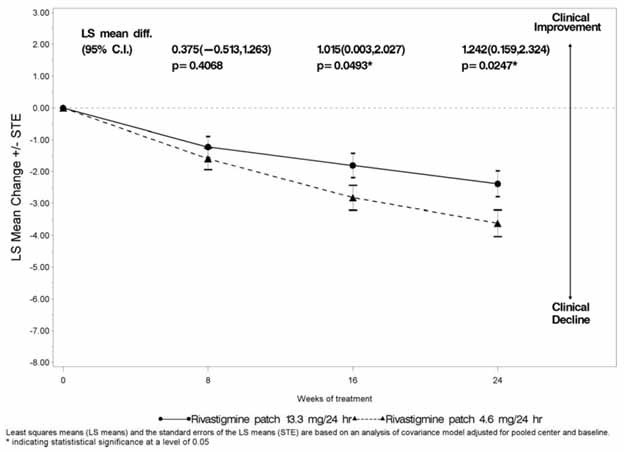
1 INDICATIONS AND USAGE Rivastigmine transdermal system is an acetylcholinesterase inhibitor indicated for treatment of: Mild, moderate, and severe dementia of the Alzheimer’s type (AD). (1.1) Mild-to-moderate dementia associated with Parkinson’s disease (PD). (1.2) 1.1 Alzheimer’s Disease Rivastigmine transdermal system is indicated for the treatment of dementia of the Alzheimer’s type (AD). Efficacy has been demonstrated in patients with mild, moderate, and severe Alzheimer’s disease. 1.2 Parkinson’s Disease Dementia Rivastigmine transdermal system is indicated for the treatment of mild-to-moderate dementia associated with Parkinson’s disease (PDD).
Dosage and Administration
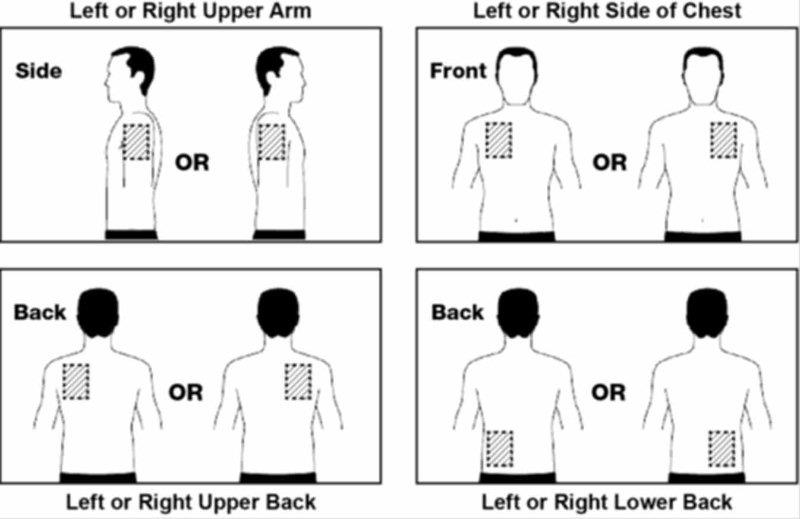
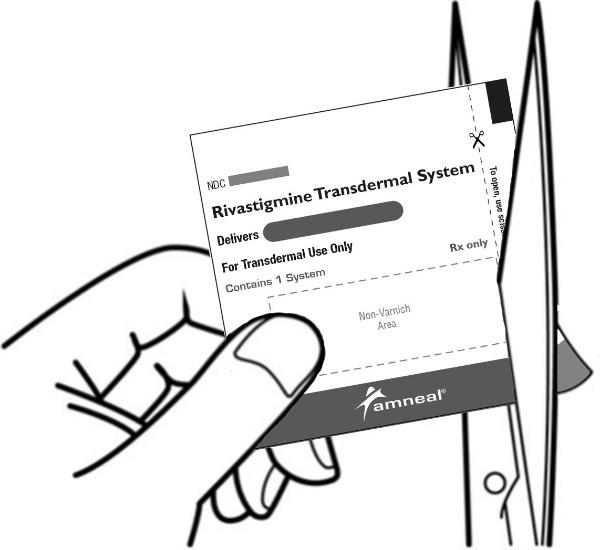
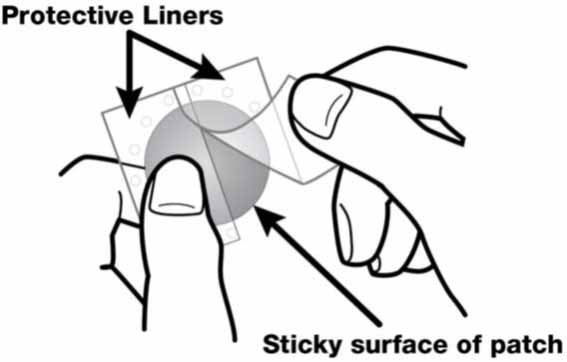
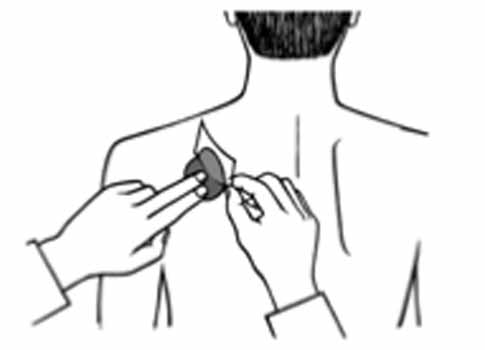
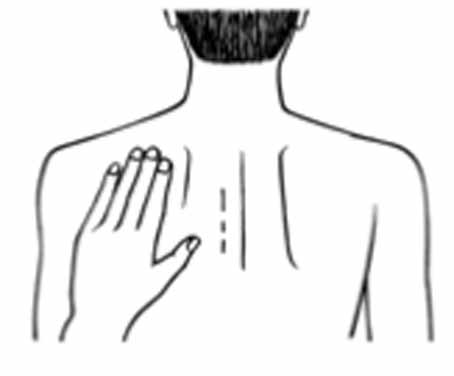
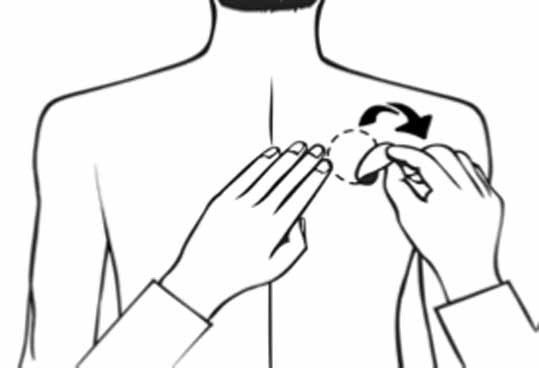
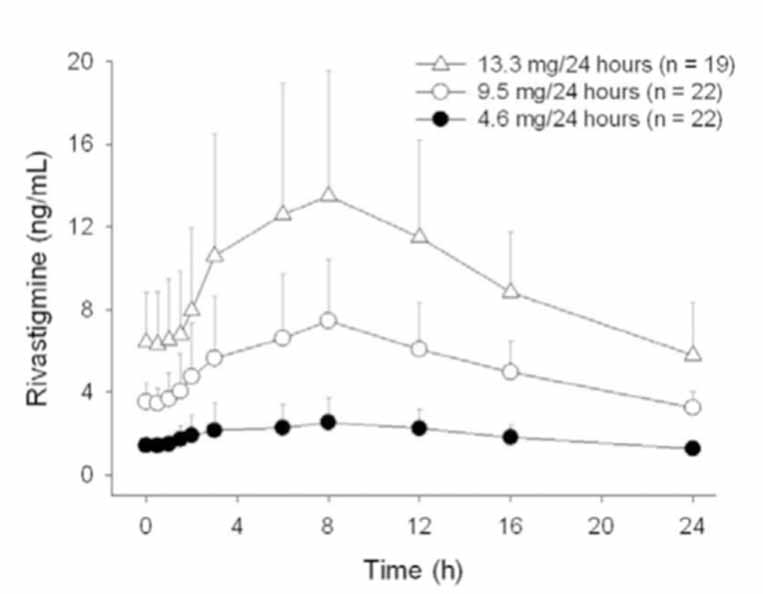
2 DOSAGE AND ADMINISTRATION Apply patch on intact skin for a 24-hour period; replace with a new patch every 24 hours. (2.1, 2.4) Initial Dose: Initiate treatment with 4.6 mg/24 hours rivastigmine transdermal system. (2.1) Dose Titration: After a minimum of 4 weeks, if tolerated, increase dose to 9.5 mg/24 hours, which is the minimum effective dose. Following a minimum additional 4 weeks, may increase dosage to maximum dosage of 13.3 mg/24 hours. (2.1) Mild-to-Moderate Alzheimer’s Disease and Parkinson’s Disease Dementia: Rivastigmine transdermal system 9.5 mg/24 hours or 13.3 mg/24 hours once daily. (2.1) Severe Alzheimer’s Disease: Rivastigmine transdermal system 13.3 mg/24 hours once daily. (2.1) For treatment interruption longer than 3 days, retitrate dosage starting at 4.6 mg per 24 hours. (2.1) Consider dose adjustments in patients with (2.2) : Mild-to-moderate hepatic impairment (8.6) Low (less than 50 kg) body weight (8.7) 2.1 Recommended Dosing Initial Dose Initiate treatment with one 4.6 mg/24 hours rivastigmine transdermal system applied to the skin once daily [see Dosage and Administration (2.4) ] . Dose Titration Increase the dose only after a minimum of 4 weeks at the previous dose, and only if the previous dose has been tolerated. For mild-to-moderate AD and PDD patients, continue the effective dose of 9.5 mg/24 hours for as long as therapeutic benefit persists. Patients can then be increased to the maximum effective dose of 13.3 mg/24 hours dose. For patients with severe AD, 13.3 mg/24 hours is the effective dose. Doses higher than 13.3 mg/24 hours confer no appreciable additional benefit, and are associated with an increase in the incidence of adverse reactions [see Warnings and Precautions (5.2) , Adverse Reactions (6.1) ] . Mild-to-Moderate Alzheimer’s Disease and Mild-to-Moderate Parkinson’s Disease Dementia The effective dosage of rivastigmine transdermal system is 9.5 mg/24 hours or 13.3 mg/24 hours administered once per day; replace with a new patch every 24 hours. Severe Alzheimer’s Disease The effective dosage of rivastigmine transdermal system in patients with severe Alzheimer’s disease is 13.3 mg/24 hours administered once per day; replace with a new patch every 24 hours. Interruption of Treatment If dosing is interrupted for 3 days or fewer, restart treatment with the same or lower strength rivastigmine transdermal system. If dosing is interrupted for more than 3 days, restart treatment with the 4.6 mg/24 hours rivastigmine transdermal system and titrate as described above. 2.2 Dosing in Specific Populations Dosing Modifications in Patients with Hepatic Impairment Consider using the 4.6 mg/24 hours rivastigmine transdermal system as both the initial and maintenance dose in patients with mild (Child-Pugh score 5 to 6) to moderate (Child-Pugh score 7 to 9) hepatic impairment [see Use in Specific Populations (8.6) , Clinical Pharmacology (12.3) ] . Dosing Modifications in Patients with Low Body Weight Carefully titrate and monitor patients with low body weight (less than 50 kg) for toxicities (e.g., excessive nausea, vomiting), and consider reducing the maintenance dose to the 4.6 mg/24 hours rivastigmine transdermal system if such toxicities develop. 2.3 Switching to Rivastigmine Transdermal System from Rivastigmine Tartrate Capsules or Rivastigmine Tartrate Oral Solution Patients treated with rivastigmine tartrate capsules or oral solution may be switched to rivastigmine transdermal system as follows: A patient who is on a total daily dose of less than 6 mg of oral rivastigmine can be switched to the 4.6 mg/24 hours rivastigmine transdermal system. A patient who is on a total daily dose of 6 mg to 12 mg of oral rivastigmine can be switched to the 9.5 mg/24 hours rivastigmine transdermal system. Instruct patients or caregivers to apply the first patch on the day following the last oral dose. 2.4 Important Administration Instructions Rivastigmine transdermal system is a patch for transdermal use on intact skin. (a) Do not use the patch if the pouch seal is broken or the patch is cut, damaged, or changed in any way. (b) Apply the rivastigmine transdermal system once a day. Press down firmly for 30 seconds until the edges stick well when applying to clean, dry, hairless, intact healthy skin in a place that will not be rubbed against by tight clothing. Use the upper or lower back as the site of application because the patch is less likely to be removed by the patient. If sites on the back are not accessible, apply the patch to the upper arm or chest. Do not apply to a skin area where cream, lotion, or powder has recently been applied. (c) Do not apply to skin that is red, irritated, or cut. (d) Replace the rivastigmine transdermal system with a new patch every 24 hours. Instruct patients to only wear 1 patch at a time (remove the previous day’s patch before applying a new patch) [see Warnings and Precautions (5.1) , Overdosage (10) ] . If a patch falls off or if a dose is missed, apply a new patch immediately, and then replace this patch the following day at the usual application time. (e) Change the site of patch application daily to minimize potential irritation, although a new patch can be applied to the same general anatomic site (e.g., another spot on the upper back) on consecutive days. Do not apply a new patch to the same location for at least 14 days. (f) May wear the patch during bathing and in hot weather. Avoid long exposure to external heat sources (excessive sunlight, saunas, solariums). (g) Place used patches in the previously saved pouch and discard in the trash, away from pets or children. (h) Wash hands with soap and water after removing the patch. In case of contact with eyes or if the eyes become red after handling the patch, rinse immediately with plenty of water, and seek medical advice if symptoms do not resolve.
