Drug Catalog - Product Detail
RILUZOLE TB 50MG 60
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 67877-0286-60 | ASCEND LABORATORIES | 60 | 50MG | TABLET |
PACKAGE FILES

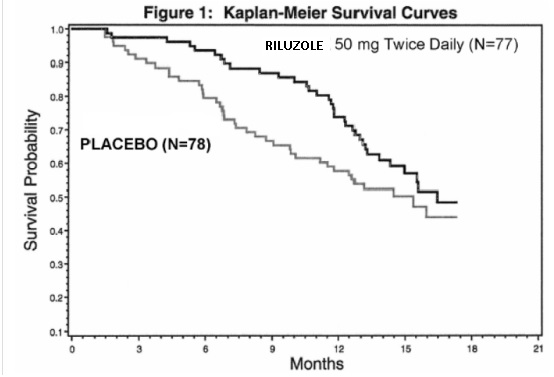
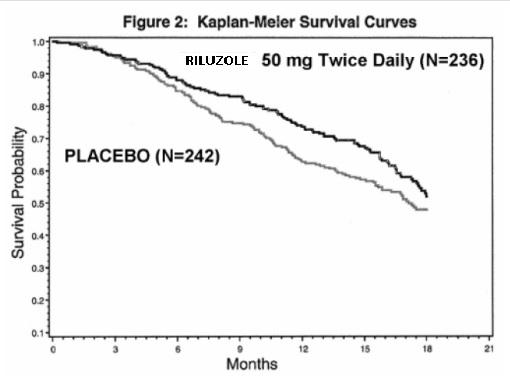
Generic Name
RILUZOLE
Substance Name
RILUZOLE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA204048
Description
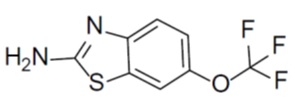
11 DESCRIPTION Riluzole is a member of the benzothiazole class. The chemical designation for riluzole is 2-amino-6-(trifluoromethoxy)benzothiazole. Its molecular formula is C 8 H 5 F 3 N 2 OS, and its molecular weight is 234.2. The chemical structure is: Riluzole is a white to slightly yellow powder that is freely soluble in acetonitrile, in alcohol, in methylene chloride, very slightly soluble in hexane and water. Riluzole Tablets, USP is available as a white to off-white coloured, capsule shaped film coated tablet, debossed with “RIL” on one side and “50” on other side. Each film-coated tablet for oral use contains 50 mg of riluzole and the following inactive ingredients: Core: dibasic calcium phosphate dihydrate, USP; croscarmellose sodium, USP/NF; hypromellose, USP; microcrystalline cellulose, USP/NF; magnesium stearate, USP/NF; colloidal silicon dioxide, USP/NF. Film coating: Opadry Y-1-7000H White (hypromellose, USP; titanium dioxide, USP; polyethylene glycol 400, NF) riluzole-st
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Riluzole Tablets, USP are white to off-white coloured, capsule-shaped film coated tablets, debossed with “RIL” on one side and “50” on other side. Riluzole Tablets, USP are supplied in the following presentations: Bottles of 60 tablets NDC 67877-286-60 Bottles of 90 tablets NDC 67877-286-90 Bottles of 500 tablets NDC 67877-286-05 Bottles of 1000 tablets NDC 67877-286-10 Blister pack of 140 (10 x 14) Tablets NDC 67877-286-14 Store at 20° to 25°C (68° to 77°F). [See USP controlled room temperature.] and protect from bright light. Keep out of the reach of children.
Indications & Usage
1 INDICATIONS & USAGE Riluzole is indicated for the treatment of amyotrophic lateral sclerosis (ALS). Riluzole is indicated for the treatment of amyotrophic lateral sclerosis (ALS) ( 1 )
Dosage and Administration
2 DOSAGE & ADMINISTRATION The recommended dosage for riluzole is 50 mg taken orally twice daily. Riluzole should be taken at least 1 hour before or 2 hours after a meal [see Clinical Pharmacology ( 12.3 )] . Measure serum aminotransferases before and during treatment with riluzole [ see Warnings and Precautions (5.1 )]. · Recommended dosage: 50 mg twice daily, taken at least 1 hour before or 2 hours after a meal ( 2 ) · Measure serum aminotransferases before and during treatment ( 2 , 5.1 )
