Drug Catalog - Product Detail
RELADOR PAK
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 69166-0105-90 | ACCELIS PHARMA | 1 | 2.5-2.5% | KIT |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
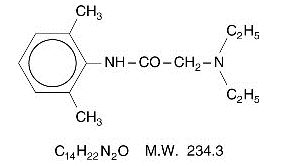
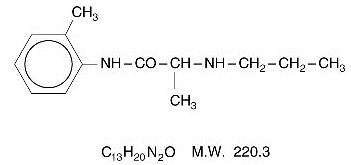
DESCRIPTION Lidocaine 2.5% and Prilocaine 2.5%, a topical anesthetic agent, is an emulsion in which the oil phase is a eutectic mixture of lidocaine and prilocaine in a ratio of 1:1 by weight. This eutectic mixture has a melting point below room temperature and therefore both local anesthetics exist as a liquid oil rather than as crystals. It is packaged in 15 gram and 30 gram tubes. Lidocaine is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl), has an octanol:water partition ratio of 43 at pH 7.4, and has the following structure: Prilocaine is chemically designated as propanamide, N-(2-methylphenyl)-2-(propylamino), has an octanol:water partition ratio of 25 at pH 7.4, and has the following structure: Each gram of lidocaine 2.5% and prilocaine 2.5% cream contains lidocaine 25 mg, prilocaine 25 mg, carboxypolymethylene (as a thickening agent), polyoxyethylene fatty acid esters (as emulsifiers), purified water to 1 gram, and sodium hydroxide to adjust pH (pH range 9.0-9.4). Lidocaine 2.5% and prilocaine 2.5% cream contains no preservative, however it passes the USP antimicrobial effectiveness test due to the pH. The specific gravity of lidocaine 2.5% and prilocaine 2.5% cream is 1.00. Lidocaine Chemical Structure Prilocaine Chemical Structure
How Supplied
HOW SUPPLIED Lidocaine 2.5% and Prilocaine 2.5% Cream is available as the following: NDC 50383-667-15 15 gram tube, box of 1 NDC 50383-667-30 30 gram tube, box of 1 NOT FOR OPHTALMIC USE. KEEP CONTAINER TIGHTLY CLOSED AT ALL TIMES WHEN NOT IN USE. Store at controlled room temperature 15° to 30°C (59° to 86°F) [see USP]. Manufactured by: Hi-Tech Pharmacal Co., Inc. Amityville, NY 11701 Rev. 667:02 7/09 MG #16518
Indications & Usage
INDICATIONS AND USAGE Lidocaine 2.5% and prilocaine 2.5% cream (a eutectic mixture of lidocaine 2.5% and prilocaine 2.5%) is indicated as a topical anesthetic for use on: normal intact skin for local analgesia. genital mucous membranes for superficial minor surgery and as pretreatment for infiltration anesthesia. Lidocaine 2.5% and prilocaine 2.5% cream is not recommended in any clinical situation when penetration or migration beyond the tympanic membrane into the middle ear is possible because of the ototoxic effects observed in animal studies (see WARNINGS ).
Dosage and Administration
DOSAGE AND ADMINISTRATION Adult Patients − Intact Skin A thick layer of lidocaine 2.5% and prilocaine 2.5% cream is applied to intact skin and covered with an occlusive dressing (see INSTRUCTIONS FOR APPLICATION: ). Minor Dermal Procedures: For minor procedures such as intravenous cannulation and venipuncture, apply 2.5 grams of lidocaine 2.5% and prilocaine 2.5% cream (1/2 the 5 g tube) over 20 to 25 cm 2 of skin surface for at least 1 hour. In controlled clinical trials using lidocaine 2.5% and prilocaine 2.5% cream, two sites were usually prepared in case there was a technical problem with cannulation or venipuncture at the first site. Major Dermal Procedures: For more painful dermatological procedures involving a larger skin area such as split thickness skin graft harvesting, apply 2 grams of lidocaine 2.5% and prilocaine 2.5% cream per 10 cm 2 of skin and allow to remain in contact with the skin for at least 2 hours. Adult Male Genital Skin: As an adjunct prior to local anesthetic infiltration, apply a thick layer of lidocaine 2.5% and prilocaine 2.5% cream (1 g/10 cm 2) to the skin surface for 15 minutes. Local anesthetic infiltration should be performed immediately after removal of lidocaine 2.5% and prilocaine 2.5% cream. Dermal analgesia can be expected to increase for up to 3 hours under occlusive dressing and persist for 1 to 2 hours after removal of the cream. The amount of lidocaine and prilocaine absorbed during the period of application can be estimated from the information in Table 2 , ** footnote, in Individualization of Dose. Adult Female Patients − Genital Mucous Membranes For minor procedures on the female external genitalia, such as removal of condylomata acuminata, as well as for use as pretreatment for anesthetic infiltration, apply a thick layer (5-10 grams) of lidocaine 2.5% and prilocaine 2.5% cream for 5 to 10 minutes. Occlusion is not necessary for absorption, but may be helpful to keep the cream in place. Patients should be lying down during the lidocaine 2.5% and prilocaine 2.5% cream application, especially if no occlusion is used. The procedure or the local anesthetic infiltration should be performed immediately after the removal of lidocaine 2.5% and prilocaine 2.5% cream. Pediatric Patients − Intact Skin The following are the maximum recommended doses, application areas and application times for lidocaine 2.5% and prilocaine 2.5% cream based on a child’s age and weight: Age and Body Weight Requirements Maximum Total Dose of lidocaine 2.5% and prilocaine 2.5% cream Maximum Application Area Maximum Application Time 0 up to 3 months or < 5 kg 1 g 10 cm2 1 hour 3 up to 12 months and > 5 kg 2 g 20 cm2 4 hours 1 to 6 years and > 10 kg 10 g 100 cm2 4 hours 7 to 12 years and > 20 kg 20 g 200 cm2 4 hours Please note: If a patient greater than 3 months old does not meet the minimum weight requirement, the maximum total dose of lidocaine 2.5% and prilocaine 2.5% cream should be restricted to that which corresponds to the patient’s weight . (see INSTRUCTIONS FOR APPLICATION ). Practitioners should carefully instruct caregivers to avoid application of excessive amounts of lidocaine 2.5% and prilocaine 2.5% cream (see PRECAUTIONS ). When applying lidocaine 2.5% and prilocaine 2.5% cream to the skin of young children, care must be taken to maintain careful observation of the child to prevent accidental ingestion of lidocaine 2.5% and prilocaine 2.5% cream or the occlusive dressing. A secondary protective covering to prevent inadvertent disruption of the application site may be useful. Lidocaine 2.5% and prilocaine 2.5% cream should not be used in neonates with a gestational age less than 37 weeks nor in infants under the age of 12 months who are receiving treatment with methemoglobin-inducing agents (see Methemoglobinemia subsection of WARNINGS ). When lidocaine 2.5% and prilocaine 2.5% cream (lidocaine 2.5% and prilocaine 2.5%) is used concomitantly with other products containing local anesthetic agents, the amount absorbed from all formulations must be considered (see Individualization of Dose ). The amount absorbed in the case of lidocaine 2.5% and prilocaine 2.5% cream is determined by the area over which it is applied and the duration of application under occlusion (see Table 2 , ** footnote, in Individualization of Dose). Although the incidence of systemic adverse reactions with lidocaine 2.5% and prilocaine 2.5% cream is very low, caution should be exercised, particularly when applying it over large areas and leaving it on for longer than 2 hours. The incidence of systemic adverse reactions can be expected to be directly proportional to the area and time of exposure (see Individualization of Dose ).
