Drug Catalog - Product Detail
RANOLAZINE ER 1000MG TB 60
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 27241-0126-02 | AJANTA PHARMA LIMITED | 60 | 1000MG | TABLET |
PACKAGE FILES


Generic Name
RANOLAZINE
Substance Name
RANOLAZINE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA210054
Description
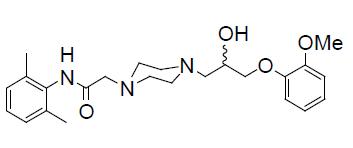
11 DESCRIPTION Ranolazine extended-release tablets are available as a film-coated, non-scored, extended-release tablet for oral administration. Ranolazine is a racemic mixture, chemically described as 1-piperazineacetamide, N -(2,6-dimethylphenyl)-4-[2-hydroxy-3-(2-methoxyphenoxy)propyl]-, (±)-. It has an molecular formula of C 24 H 33 N 3 O 4 , a molecular weight of 427.54 g/mole, and the following structural formula: Ranolazine is a white to off-white powder. Ranolazine is soluble in methanol; sparingly soluble in acetonitrile; slightly soluble in isopropyl alcohol and very slightly soluble in water. Ranolazine extended-release tablets contain 500 mg or 1000 mg of ranolazine and the following inactive ingredients: Hypromellose, magnesium stearate, methacrylic acid and ethyl acrylate copolymer (sodium lauryl sulfate and polysorbate 80), microcrystalline cellulose, polyethylene glycol and sodium hydroxide. The color coating contains titanium dioxide, polyvinyl alcohol, talc, hypromellose, polyethylene glycol, Iron Oxide Yellow, and Iron Oxide Red. structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Ranolazine extended-release tablets are supplied as film-coated, oval-shaped, biconvex, extended-release tablets in the following strengths: 500 mg tablets are orange colored, with RZ1 on one side and plain on other side 1000 mg tablets are yellow colored, with RZ2 on one side and plain on other side Ranolazine extended-release tablets are available in: Strength NDC Unit-of-Use Bottle (60 Tablets) 500 mg 27241-125-02 Bottle (500 Tablets) 500 mg 27241-125-05 Unit-of-Use Bottle (60 Tablets) 1000 mg 27241-126-02 Bottle (500 Tablets) 1000 mg 27241-126-05 Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F). [See USP Controlled Room Temperature.]
Indications & Usage
1 INDICATIONS AND USAGE Ranolazine extended-release tablets are indicated for the treatment of chronic angina. Ranolazine extended-release tablets may be used with beta-blockers, nitrates, calcium channel blockers, anti-platelet therapy, lipid-lowering therapy, ACE inhibitors, and angiotensin receptor blockers. Ranolazine is an antianginal indicated for the treatment of chronic angina. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION 500 mg twice daily and increase to 1000 mg twice daily, based on clinical symptoms ( 2.1 ) 2.1 Dosing Information Initiate ranolazine extended-release tablets dosing at 500 mg twice daily and increase to 1000 mg twice daily, as needed, based on clinical symptoms. Take ranolazine extended-release tablets with or without meals. Swallow Ranolazine extended-release tablets whole; do not crush, break, or chew. The maximum recommended daily dose of ranolazine extended-release tablets is 1000 mg twice daily. If a dose of ranolazine extended-release tablets is missed, take the prescribed dose at the next scheduled time; do not double the next dose. 2.2 Dose Modification Dose adjustments may be needed when ranolazine extended-release tablets are taken in combination with certain other drugs [see Drug Interactions ( 7.1 )] . Limit the maximum dose of ranolazine extended-release tablets to 500 mg twice daily in patients on moderate CYP3A inhibitors such as diltiazem, verapamil, and erythromycin. Use of ranolazine extended-release tablets with strong CYP3A inhibitors is contraindicated [see Contraindications ( 4 ), Drug Interactions ( 7.1 )] . Use of P-gp inhibitors, such as cyclosporine, may increase exposure to ranolazine extended-release tablets. Titrate ranolazine extended-release tablets based on clinical response [see Drug Interactions ( 7.1 )] .
