Drug Catalog - Product Detail
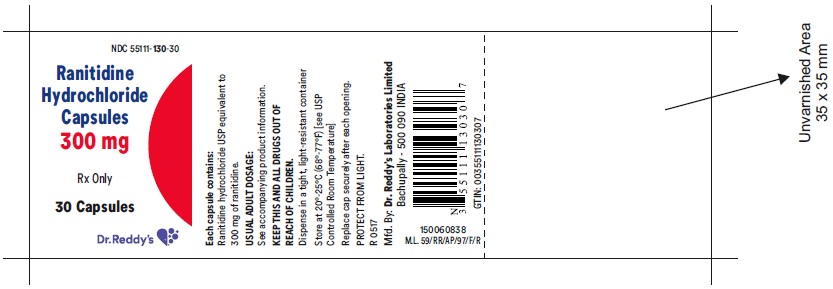
RANITIDINE HCL (GELDOSE) CP 300MG 30
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 55111-0130-30 | DR.REDDY'S LABORATORIES, INC. | 30 | 300MG | CAPSULE |
PACKAGE FILES
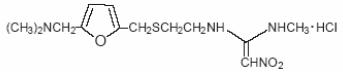
Generic Name
RANITIDINE HYDROCHLORIDE
Substance Name
RANITIDINE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA075742
Description
DESCRIPTION Ranitidine hydrochloride (HCl) USP, is a histamine H 2 -receptor antagonist. Chemically it is N-[2-[[[5-[(dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-N’-methyl-2-nitro-1,1-ethenediamine, HCl. It has the following structural formula: The molecular formula is C 13 H 22 N 4 O 3 S•HCl, representing a molecular weight of 350.87. Ranitidine hydrochloride USP is a white to pale yellow, crystalline practically odorless powder, is sensitive to light and moisture. It is very soluble in water and sparingly soluble in alcohol. Each Ranitidine Hydrochloride capsule, for oral administration, contains 167.4 mg or 334.8 mg of ranitidine hydrochloride equivalent to 150 mg or 300 mg of ranitidine, respectively. In addition, each capsule contains the following inactive ingredients: Microcrystalline Cellulose, Sodium Starch Glycolate, Magnesium Stearate. The capsule shells contain Black Iron Oxide, Red Iron Oxide T3469, Yellow Iron Oxide T3506, Titanium Dioxide and Gelatin. The capsule shells are imprinted with edible ink.
How Supplied
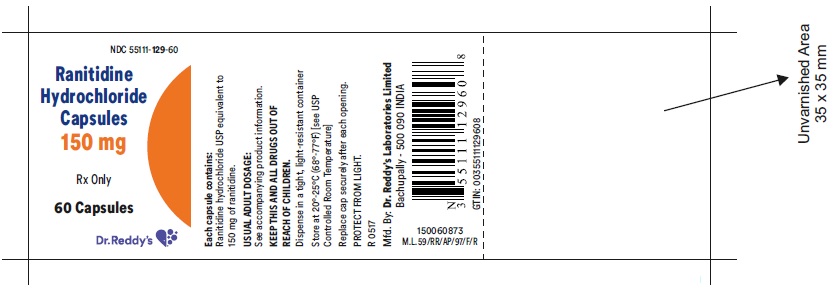
HOW SUPPLIED Ranitidine Hydrochloride Capsules 150 mg White to pale yellow powder filled in size '3', hard gelatin capsules with opaque light brown colored cap and opaque light brown colored body, imprinted 'CD' on cap and '129' on body, with black ink. The capsules are supplied in bottles of 60's and 500's. Bottles of 60 NDC 55111-129-60 Bottles of 500 NDC 55111-129-05 Ranitidine Hydrochloride Capsules 300 mg White to pale yellow powder filled in size '1', hard gelatin capsules with opaque light brown colored cap and opaque light brown colored body, imprinted 'CD' on cap and '130' on body, with black ink. The capsules are supplied in bottles of 30's and 100's. Bottles of 30 NDC55111-130-30 Bottles of 100 NDC 55111-130-01 Store at 20°-25°C (68°-77°F) [see USP Controlled Room Temperature] in a dry place. Protect from light. Dispense in a tight, light resistant container. Rx Only Manufactured by: Dr. Reddy’s Laboratories Limited Bachupally – 500 090 INDIA Revised: 0517
Indications & Usage
INDICATIONS AND USAGE Ranitidine is indicated in: 1. Short-term treatment of active duodenal ulcer. Most patients heal within 4 weeks. Studies available to date have not assessed the safety of ranitidine in uncomplicated duodenal ulcer for periods of more than 8 weeks. 2. Maintenance therapy for duodenal ulcer patients at reduced dosage after healing of acute ulcers. No placebo-controlled comparative studies have been carried out for periods of longer than 1 year. 3.The treatment of pathological hypersecretory conditions (e.g., Zollinger-Ellison syndrome and systemic mastocytosis). 4. Short-term treatment of active, benign gastric ulcer. Most patients heal within 6 weeks and the usefulness of further treatment has not been demonstrated. Studies available to date have not assessed the safety of ranitidine in uncomplicated, benign gastric ulcer for periods of more than 6 weeks. 5.Maintenance therapy for gastric ulcer patients at reduced dosage after healing of acute ulcers. Placebo-controlled studies have been carried out for 1 year. 6.Treatment of GERD. Symptomatic relief commonly occurs within 24 hours after starting therapy with ranitidine 150 mg two times a day. 7. Treatment of endoscopically diagnosed erosive esophagitis. Symptomatic relief of heartburn commonly occurs within 24 hours of therapy initiation with ranitidine 150 mg 4 times daily. 8. Maintenance of healing of erosive esophagitis. Placebo-controlled trials have been carried out for 48 weeks. Concomitant antacids should be given as needed for pain relief to patients with active duodenal ulcer; active, benign gastric ulcer; hypersecretory states; GERD; and erosive esophagitis.
Dosage and Administration
DOSAGE AND ADMINISTRATION Active Duodenal Ulcer: The current recommended adult oral dosage of ranitidine for duodenal ulcer is 150 mg twice daily. An alternative dosage of 300 mg once daily after the evening meal or at bedtime can be used for patients in whom dosing convenience is important. The advantages of one treatment regimen compared to the other in a particular patient population have yet to be demonstrated (see CLINICAL PHARMACOLOGY : Clinical Trials : Active Duodenal Ulcer ). Smaller doses have been shown to be equally effective in inhibiting gastric acid secretion in US studies, and several foreign trials have shown that 100 mg twice daily is as effective as the 150 mg dose. Antacid should be given as needed for relief of pain (see CLINICAL PHARMACOLOGY : Pharmacokinetics ) . Maintenance of Healing Duodenal Ulcers The current recommended adult oral dosage is 150 mg at bedtime. Pathological Hypersecretory Conditions (such as Zollinger-Ellison syndrome) The current recommended adult oral dosage is 150 mg twice a day. In some patients it may be necessary to administer ranitidine 150 mg doses more frequently. Dosages should be adjusted to individual patient needs, and should continue as long as clinically indicated. Dosages up to 6 g/day have been employed in patients with severe disease. Benign Gastric Ulcer The current recommended adult oral dosage is 150 mg twice a day. Maintenance of Healing of Gastric Ulcers The current recommended adult oral dosage is 150 mg at bedtime. GERD The current recommended adult oral dosage is 150 mg twice a day. Erosive Esophagitis The current recommended adult oral dosage is 150 mg four times a day. Maintenance of Healing of Erosive Esophagitis The current recommended adult oral dosage is 150 mg twice a day. Pediatric Use The safety and effectiveness of ranitidine has been established in the age-group of 1 month to 16 years. There is insufficient information about the pharmacokinetics of ranitidine in neonatal patients (less than 1 month of age) to make dosing recommendations. The following 3 subsections provide dosing information for each of the pediatric indications. Treatment of Duodenal and Gastric Ulcers The recommended oral dose for the treatment of active duodenal and gastric ulcers is 2 to 4 mg/kg twice daily to a maximum of 300 mg/day. This recommendation is derived from adult clinical studies and pharmacokinetic data in pediatric patients. Maintenance of Healing of Duodenal and Gastric Ulcers The recommended oral dose for the maintenance of healing of duodenal and gastric ulcers is 2 to 4 mg/kg once daily to a maximum of 150 mg/day. This recommendation is derived from adult clinical studies and pharmacokinetic data in pediatric patients. Treatment of GERD and Erosive Esophagitis Although limited data exist for these conditions in pediatric patients, published literature supports a dosage of 5 to 10 mg/kg per day, usually given as two divided doses. Dosage Adjustment for Patients with Impaired Renal Function On the basis of experience with a group of subjects with severely impaired renal function treated with ranitidine, the recommended dosage in patients with a creatinine clearance <50 mL/min is 150 mg every 24 hours. Should the patient’s condition require, the frequency of dosing may beincreased to every 12 hours or even further with caution. Hemodialysis reduces the level of circulating ranitidine. Ideally, the dosing schedule should be adjusted so that the timing of a scheduled dose coincides with the end of hemodialysis. Elderly patients are more likely to have decreased renal function, therefore caution should be exercised in dose selection, and it may be useful to monitor renal function (see CLINICAL PHARMACOLOGY : Pharmacokinetics : Geriatric Use and PRECAUTIONS : Geriatric Use ).
