Drug Catalog - Product Detail
QUININE SULFATE CAP 324 MG 30 CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68180-0560-06 | LUPIN PHARMACEUTICALS | 30 | 324MG | CAPSULE |
PACKAGE FILES
Generic Name
QUININE SULFATE
Substance Name
QUININE SULFATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA203112
Description
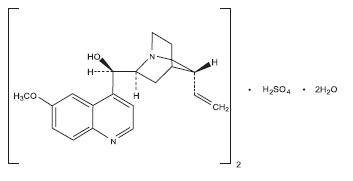
11 DESCRIPTION Quinine sulfate is a cinchona alkaloid chemically described as cinchonan-9-ol, 6'-methoxy-, (8α, 9R)-, sulfate (2:1) (salt), dihydrate with a molecular formula of (C 20 H 24 N 2 O 2 ) 2 •H 2 SO 4 •2H 2 O and a molecular weight of 782.94. The structural formula of quinine sulfate is: Quinine sulfate occurs as a white, crystalline powder that darkens on exposure to light. It is odorless and has a persistent very bitter taste. It is only slightly soluble in water, alcohol, chloroform, and ether. Quinine sulfate capsules USP are supplied for oral administration as capsules containing 324 mg of the active ingredient quinine sulfate USP, equivalent to 269 mg free base. Inactive ingredients: black iron oxide, corn starch, gelatin, magnesium stearate, potassium hydroxide, propylene glycol shellac, sodium lauryl sulfate, and talc. 1
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied Quinine sulfate capsules USP, 324 mg are available as size "0" capsules with clear transparent cap and clear transparent body imprinted with "LU" on cap and "Y51" on body in black ink, containing white to off white powder: Bottles of 30 NDC 68180-560-06 Bottles of 100 NDC 68180-560-01 Bottles of 500 NDC 68180-560-02 Bottles of 1000 NDC 68180-560-03 16.2 Storage Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature] Dispense in a tight container as defined in the USP.
Indications & Usage
1 INDICATIONS AND USAGE Quinine sulfate capsule USP is a cinchona alkaloid indicated for treatment of uncomplicated Plasmodium falciparum malaria ( 1 ). Quinine sulfate capsule USP is an antimalarial drug indicated only for treatment of uncomplicated Plasmodium falciparum malaria. Quinine sulfate has been shown to be effective in geographical regions where resistance to chloroquine has been documented [see CLINICAL STUDIES ( 14 )] . Quinine sulfate capsules USP are not approved for: Treatment of severe or complicated P. falciparum malaria. Prevention of malaria. Treatment or prevention of nocturnal leg cramps [see WARNINGS AND PRECAUTIONS ( 5.1 )] .
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Adults (≥ 16 years of age): 648 mg (two capsules) every 8 hours for 7 days ( 2.1 ). Patients with severe chronic renal impairment: one loading dose of 648 mg (two capsules) followed 12 hours later by 324 mg (one capsule) every 12 hours for 7 days ( 2.2 ). 2.1 Treatment of Uncomplicated P. falciparum Malaria For treatment of uncomplicated P. falciparum malaria in adults: Orally, 648 mg (two capsules) every 8 hours for 7 days [see CLINICAL STUDIES ( 14 )] . Quinine sulfate capsules USP should be taken with food to minimize gastric upset [see CLINICAL PHARMACOLOGY ( 12.3 )] . 2.2 Renal Impairment In patients with acute uncomplicated malaria and severe chronic renal impairment, the following dosage regimen is recommended: one loading dose of 648 mg quinine sulfate capsules USP followed 12 hours later by maintenance doses of 324 mg every 12 hours. The effects of mild and moderate renal impairment on the safety and pharmacokinetics of quinine sulfate are not known [see USE IN SPECIFIC POPULATIONS ( 8.6 ), CLINICAL PHARMACOLOGY ( 12.3 )] . 2.3 Hepatic Impairment Adjustment of the recommended dose is not required in mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment, but patients should be monitored closely for adverse effects of quinine. Quinine should not be administered in patients with severe (Child-Pugh C) hepatic impairment [see USE IN SPECIFIC POPULATIONS ( 8.7 ), CLINICAL PHARMACOLOGY ( 12.3 )] .
