Drug Catalog - Product Detail
PYRIDOSTIGMINE BROMIDE TB 60MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00115-3511-01 | AMNEAL PHARMACEUTICALS | 100 | 60MG | TABLET |
PACKAGE FILES

Generic Name
PYRIDOSTIGMINE BROMIDE
Substance Name
PYRIDOSTIGMINE BROMIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA040502
Description
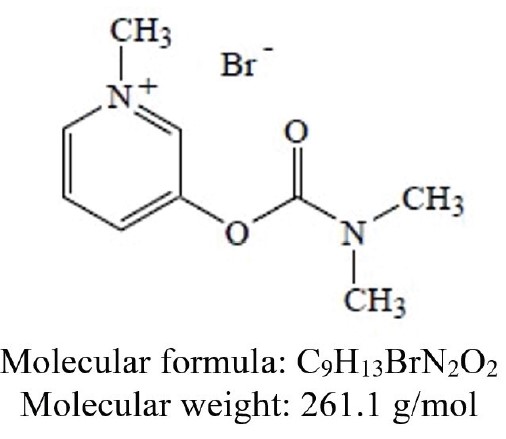
DESCRIPTION Pyridostigmine bromide, USP is an orally active cholinesterase inhibitor. Chemically, pyridostigmine bromide is 3-hydroxy-1-methylpyridinium bromide dimethylcarbamate. Its structural formula is: Pyridostigmine bromide tablets, USP is available as a 60 mg tablet for oral administration. The tablet contains the following inactive ingredients: colloidal silicon dioxide, lactose anhydrous, magnesium stearate and stearic acid. MF
How Supplied
HOW SUPPLIED Pyridostigmine Bromide Tablets USP, 60 mg are white to off white, flat-faced, round tablets debossed with "G” and “3511" on one side and double-scored on the other side. They are available as follows: Bottles of 100: NDC 0115-3511-01 Bottles of 500: NDC 0115-3511-02 IMPORTANT: These tablets are hygroscopic. Keep in a dry place with the silica gel enclosed. Dispense in a tightly-closed, light-resistant container as defined in the USP, with a child-resistant closure, as required. Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Indications & Usage
INDICATIONS AND USAGE Pyridostigmine bromide tablets are useful in the treatment of myasthenia gravis.
Dosage and Administration
DOSAGE AND ADMINISTRATION Pyridostigmine bromide is available in tablets, each containing 60 mg pyridostigmine bromide. Dosage The size and frequency of the dosage must be adjusted to the needs of the individual patient. The average dose is ten 60-mg tablets daily, spaced to provide maximum relief when maximum strength is needed. In severe cases as many as 25 tablets a day may be required, while in mild cases one to six tablets a day may suffice. Note: For information on a diagnostic test for myasthenia gravis, and for the evaluation and stabilization of therapy, please see product literature on Tensilon ® (edrophonium chloride).
