Drug Catalog - Product Detail
PYRIDOSTIGMINE BROMIDE 180MG ER TAB 30CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00115-1404-08 | AMNEAL PHARMACEUTICALS | 30 | 180MG | TABLET |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
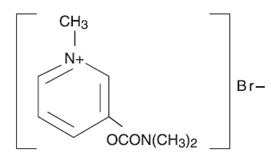
DESCRIPTION Pyridostigmine bromide is an orally active cholinesterase inhibitor. Chemically, pyridostigmine bromide is 3-hydroxy-1-methylpyridinium bromide dimethylcarbamate. Its structural formula is: Pyridostigmine bromide extended-release tablets are available as extended-release tablets containing 180 mg pyridostigmine bromide; each tablet also contains carnauba wax, copovidone, lactose, magnesium stearate, and silicon dioxide. 1
How Supplied
HOW SUPPLIED Pyridostigmine Bromide Extended-Release Tablets, 180 mg are available as light brown to pale yellow, capsule-shaped tablets, debossed with “W1” on one side and single-scored on the other side. They are supplied as follows: Bottles of 30: NDC 0115-1404-08 Note: Because of the hygroscopic nature of the extended-release tablets, mottling may occur. This does not affect their efficacy. Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Dispense in a tight, light-resistant container. Keep pyridostigmine bromide extended-release tablets in a dry place with the silica gel enclosed.
Indications & Usage
INDICATIONS AND USAGE Pyridostigmine bromide is useful in the treatment of myasthenia gravis.
Dosage and Administration
DOSAGE AND ADMINISTRATION Pyridostigmine bromide is available in extended-release dosage form: Extended-Release Tablets — each containing 180 mg pyridostigmine bromide. This form provides uniformly slow release, hence prolonged duration of drug action; it facilitates control of myasthenic symptoms with fewer individual doses daily. The immediate effect of a 180 mg extended-release tablet is about equal to that of a 60 mg immediate-release tablet; however, its duration of effectiveness, although varying in individual patients, averages 2 1/2 times that of a 60 mg dose. Dosage: The size and frequency of the dosage must be adjusted to the needs of the individual patient. Extended-Release Tablets — One to three 180 mg tablets, once or twice daily, will usually be sufficient to control symptoms; however, the needs of certain individuals may vary markedly from this average. The interval between doses should be at least 6 hours. For optimum control, it may be necessary to use the more rapidly acting regular tablets or syrup in conjunction with extended-release therapy. Note: For information on a diagnostic test for myasthenia gravis, and for the evaluation and stabilization of therapy, please see product literature on edrophonium chloride.
