Drug Catalog - Product Detail
PROPAFENONE HCL CAP ER 12HR 225 MG 60 CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68462-0408-60 | GLENMARK PHARMACEUTICALS | 60 | 225MG | CAPSULE |
PACKAGE FILES





Generic Name
PROPAFENONE HYDROCHLORIDE
Substance Name
PROPAFENONE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA205268
Description
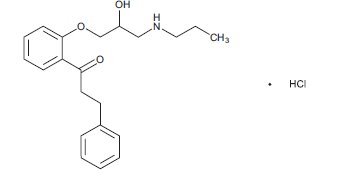
11 DESCRIPTION Propafenone Hydrochloride Extended-Release Capsules USP are an antiarrhythmic drug supplied in extended-release capsules of 225 mg, 325 mg and 425 mg for oral administration. Chemically, propafenone hydrochloride, USP is 2’-[2-hydroxy-3-(propylamino)-propoxy]-3-phenylpropiophenone hydrochloride, with a molecular weight of 377.9 g/mol. The molecular formula is C 21 H 27 NO 3 •HCl. Propafenone hydrochloride, USP has some structural similarities to beta-blocking agents. The structural formula of propafenone hydrochloride, USP is given below: Propafenone hydrochloride, USP occurs as a white to almost white powder or crystals. It is soluble in methanol and in hot water; slightly soluble in alcohol and in chloroform; very slightly soluble in acetone; insoluble in diethyl ether and in toluene. Propafenone Hydrochloride Extended-Release Capsules USP are filled with white to off white colored, round shaped flat mini tablets, containing propafenone hydrochloride, USP and the following inactive ingredients: black iron oxide, gelatin, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, potassium hydroxide, shellac, sodium lauryl sulfate and titanium dioxide. USP dissolution test is pending. Structure.jpg
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Propafenone Hydrochloride Extended-Release Capsules USP are supplied as hard gelatin capsules containing either 225 mg, 325 mg or 425 mg of propafenone hydrochloride, USP. The 225 mg capsule has a white opaque body imprinted with “408” in black ink and white opaque cap imprinted with Glenmark logo “G” in black ink. They are available as follows: NDC 68462-408-60 Bottles of 60 capsules The 325 mg capsule has a white opaque body imprinted with “409” in black ink and white opaque cap imprinted with Glenmark logo “G” in black ink. They are available as follows: NDC 68462-409-60 Bottles of 60 capsules The 425 mg capsule has a white opaque body imprinted with “410” and single band in black ink and white opaque cap imprinted with Glenmark logo “G” and single band in black ink. They are available as follows: NDC 68462-410-60 Bottles of 60 capsules Storage: Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Dispense in a tight container as defined in the USP.
Indications & Usage
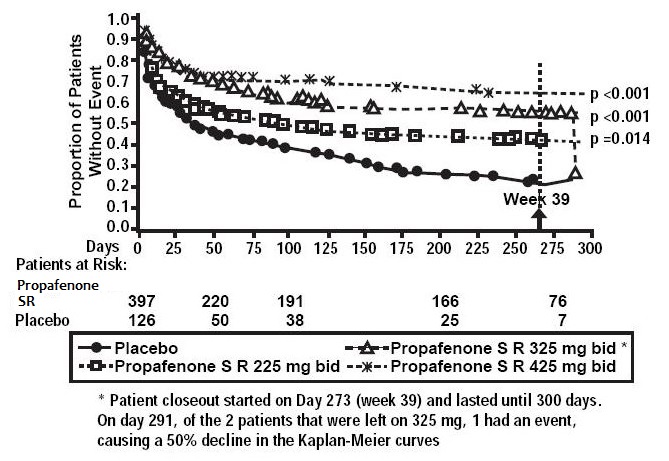
1 INDICATIONS AND USAGE Propafenone hydrochloride extended-release capsules are indicated to prolong the time to recurrence of symptomatic atrial fibrillation (AF) in patients with episodic (most likely paroxysmal or persistent) AF who do not have structural heart disease. Usage Considerations: • The use of propafenone hydrochloride extended-release capsules in patients with permanent AF or in patients exclusively with atrial flutter or paroxysmal supraventricular tachycardia (PSVT) has not been evaluated. Do not use propafenone hydrochloride extended-release capsules to control ventricular rate during AF. • Some patients with atrial flutter treated with propafenone have developed 1:1 conduction, producing an increase in ventricular rate. Concomitant treatment with drugs that increase the functional atrioventricular (AV) nodal refractory period is recommended. • The effect of propafenone on mortality has not been determined [see Boxed Warning] . Propafenone hydrochloride extended-release capsules are an antiarrhythmic indicated to prolong the time to recurrence of symptomatic atrial fibrillation (AF) in patients with episodic (most likely paroxysmal or persistent) AF who do not have structural heart disease. ( 1 ) Usage Considerations: • Use in patients with permanent atrial fibrillation or with atrial flutter or paroxysmal supraventricular tachycardia (PSVT) has not been evaluated. Do not use to control ventricular rate during atrial fibrillation. ( 1 ) • In patients with atrial fibrillation and atrial flutter, use propafenone hydrochloride extended-release capsules with drugs that increase the atrioventricular nodal refractory period. ( 1 ) • The effect of propafenone on mortality has not been determined. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Propafenone hydrochloride extended-release capsules can be taken with or without food. Do not crush or further divide the contents of the capsule. The dose of propafenone hydrochloride extended-release capsules must be individually titrated on the basis of response and tolerance. Initiate therapy with propafenone hydrochloride extended-release capsules 225 mg given every 12 hours. Dosage may be increased at a minimum of 5-day intervals to 325 mg given every 12 hours. If additional therapeutic effect is needed, the dose of propafenone hydrochloride extended-release capsules may be increased to 425 mg given every 12 hours. In patients with hepatic impairment or those with significant widening of the QRS complex or second- or third-degree AV block, consider reducing the dose. The combination of cytochrome P450 3A4 (CYP3A4) inhibition and either cytochrome P450 2D6 (CYP2D6) deficiency or CYP2D6 inhibition with the simultaneous administration of propafenone may significantly increase the concentration of propafenone and thereby increase the risk of proarrhythmia and other adverse events. Therefore, avoid simultaneous use of propafenone hydrochloride extended-release capsules with both a CYP2D6 inhibitor and a CYP3A4 inhibitor [see Warnings and Precautions ( 5.4 ), Drug Interactions ( 7.1 )]. • Initiate therapy with 225 mg given every 12 hours. ( 2 ) • Dosage may be increased at a minimum of 5-day intervals to 325 mg every 12 hours and, if necessary, to 425 mg every 12 hours. ( 2 ) • Consider reducing the dose in patients with hepatic impairment, significant widening of the QRS complex, or second- or third-degree AV block. ( 2 )
