Drug Catalog - Product Detail
PROGESTERONE MICRONIZED ORAL CAPSULE 100MG 100CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 69452-0148-20 | BIONPHARMA | 100 | 100MG | CAPSULE |
PACKAGE FILES
Generic Name
PROGESTERONE
Substance Name
PROGESTERONE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA200900
Description
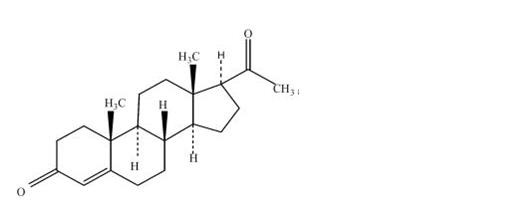
DESCRIPTION Progesterone USP, capsules contains micronized progesterone for oral administration. Progesterone has a molecular weight of 314.47 and a molecular formula of C 21 H 30 O 2 . Progesterone (pregn-4-ene-3, 20-dione) is a white or creamy white, odorless, crystalline powder practically insoluble in water, soluble in alcohol, acetone and dioxane and sparingly soluble in vegetable oils, stable in air, melting between 126° and 131°C. The structural formula is: Progesterone is synthesized from a starting material from a plant source and is chemically identical to progesterone of human ovarian origin. Progesterone capsules are available in multiple strengths to afford dosage flexibility for optimum management. Progesterone capsules contain 100 mg or 200 mg micronized progesterone. The inactive ingredients for progesterone, capsules 100 mg include: ferric oxide red NF, ferric oxide yellow NF, gelatin NF, glycerin USP, lecithin NF, peanut oil NF, titanium dioxide USP. The inactive ingredients for progesterone, capsules 200 mg include: ferric oxide red NF, gelatin NF, glycerin USP, lecithin NF, peanut oil NF, titanium dioxide USP. chemical structure
How Supplied
HOW SUPPLIED Progesterone, capsules 100 mg are available as an oval orange, opaque, capsule imprinted with P-1 in black ink. NDC 69452-148-20 (Bottle of 100) Progesterone, capsules 200 mg are available as an oval red, opaque, capsule imprinted with P-2 in black ink. NDC 69452-149-20 (Bottle of 100) Store at 20-25°C (68-77°F). [See USP Controlled Room Temperature] Protect from excessive moisture Keep out of reach of children. Dispense in tight, light-resistant container as defined in USP/NF, accompanied by a Patient Insert. Manufactured for Bionpharma Inc ., 600 Alexander Road, Princeton, NJ 08540, USA 12/2015
Indications & Usage
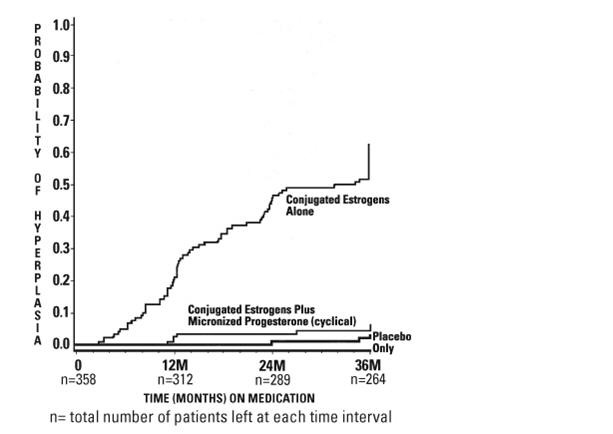
INDICATIONS AND USAGE Progesterone capsules are indicated for use in the prevention of endometrial hyperplasia in nonhysterectomized postmenopausal women who are receiving conjugated estrogens tablets. They are also indicated for use in secondary amenorrhea.
Dosage and Administration
DOSAGE AND ADMINISTRATION Prevention of Endometrial Hyperplasia Progesterone capsules should be given as a single daily dose at bedtime, 200 mg orally for 12 days sequentially per 28-day cycle, to postmenopausal women with a uterus who are receiving daily conjugated estrogens tablets. Treatment of Secondary Amenorrhea Progesterone capsules may be given as a single daily dose of 400 mg at bedtime for 10 days. Some women may experience difficulty swallowing progesterone capsules. For these women, progesterone capsules should be taken with a glass of water while in the standing position.
