Drug Catalog - Product Detail
PREGABALIN CAPS 25MG 90CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 64980-0410-09 | RISING PHARMACEUTICALS | 90 | 25MG | CAPSULE |
PACKAGE FILES








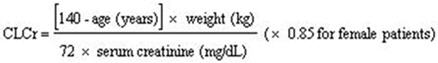
Generic Name
PREGABALIN
Substance Name
PREGABALIN
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA210432
Description
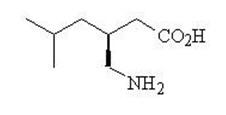
11 DESCRIPTION Pregabalin is described chemically as ( S )-3-(aminomethyl)-5-methylhexanoic acid. The molecular formula is C 8 H 17 NO 2 and the molecular weight is 159.23. The chemical structure of pregabalin is: Pregabalin is a white or almost white powder with a pK a1 of 4.4 and a pK a2 of 10.1. It is freely soluble in water and both basic and acidic aqueous solutions. The log of the partition coefficient (Octanol : Water) is – 1.0. Pregabalin capsules are administered orally and are supplied as imprinted hard-shell capsules containing 25, 50, 75, 100, 150, 200, 225, and 300 mg of pregabalin, along with pregelatinized starch and talc as inactive ingredients. The capsule shells contain gelatin, sodium lauryl sulfate and titanium dioxide. In addition, the orange & red capsule shells contain red iron oxide. The imprinting ink contains shellac, black iron oxide, propylene glycol, and potassium hydroxide. pregabalinchemistrystructure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING 25 mg capsules: White opaque/white opaque size "4" hard gelatin capsules imprinted with "LA" on cap and "41" on body with black ink, filled with white to off-white granular powder; available in: Bottles of 30: NDC 76420-117-30 (repackaged from NDC 64980-410-09) Bottles of 60: NDC 76420-117-60 (repackaged from NDC 64980-410-09) Bottles of 90: NDC 76420-117-90 (relabeled from NDC 64980-410-09) 50 mg capsules: White opaque/white opaque size "3" hard gelatin capsules imprinted with "LA" on cap and "42", black band on body with black ink, filled with white to off-white granular powder; available in: Bottles of 30: NDC 76420-118-30 (repackaged from NDC 64980-411-09) Bottles of 60: NDC 76420-118-60 (repackaged from NDC 64980-411-09) Bottles of 90: NDC 76420-118-90 (relabeled from NDC 64980-411-09) 75 mg capsules: Red opaque/white opaque size "4" hard gelatin capsules imprinted with "LA" on cap and "43" on body with black ink, filled with white to off-white granular powder; available in: Bottles of 30: NDC 76420-119-30 (repackaged from NDC 64980-412-09) Bottles of 60: NDC 76420-119-60 (repackaged from NDC 64980-412-09) Bottles of 90: NDC 76420-119-90 (relabeled from NDC 64980-412-09) 100 mg capsules: Red opaque/red opaque size "3" hard gelatin capsules imprinted with "LA" on cap and "44" on body with black ink, filled with white to off-white granular powder; available in: Bottles of 30: NDC 76420-120-30 (repackaged from NDC 64980-413-09) Bottles of 60: NDC 76420-120-60 (repackaged from NDC 64980-413-09) Bottles of 90: NDC 76420-120-90 (relabeled from NDC 64980-413-09) 150 mg capsules: White opaque/white opaque size "2" hard gelatin capsules imprinted with "LA" on cap and "45" on body with black ink, filled with white to off-white granular powder; available in: Bottles of 30: NDC 76420-121-30 (repackaged from NDC 64980-414-09) Bottles of 60: NDC 76420-121-60 (repackaged from NDC 64980-414-09) Bottles of 90: NDC 76420-121-90 (relabeled from NDC 64980-414-09) 200 mg capsules: Orange opaque/orange opaque size "1" hard gelatin capsules imprinted with "LA" on cap and "46" on body with black ink, filled with white to off-white granular powder; available in: Bottles of 30: NDC 76420-122-30 (repackaged from NDC 64980-415-09) Bottles of 60: NDC 76420-122-60 (repackaged from NDC 64980-415-09) Bottles of 90: NDC 76420-122-90 (relabeled from NDC 64980-415-09) 225 mg capsules: Orange opaque/white opaque size "1" hard gelatin capsules imprinted with "LA" on cap and "47" on body with black ink, filled with white to off-white granular powder; available in: Bottles of 30: NDC 76420-123-30 (repackaged from NDC 64980-416-09) Bottles of 60: NDC 76420-123-60 (repackaged from NDC 64980-416-09) Bottles of 90: NDC 76420-123-90 (relabeled from NDC 64980-416-09) 300 mg capsules: Red opaque/white opaque size "0" hard gelatin capsules imprinted with "LA" on cap and "48" on body with black ink, filled with white to off-white granular powder; available in: Bottles of 30: NDC 76420-124-30 (repackaged from NDC 64980-417-09) Bottles of 60: NDC 76420-124-60 (repackaged from NDC 64980-417-09) Bottles of 90: NDC 76420-124-90 (relabeled from NDC 64980-417-09) Storage and Handling Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature.] Dispense in tight container.
Indications & Usage
1 INDICATIONS AND USAGE Pregabalin capsules are indicated for: Management of neuropathic pain associated with diabetic peripheral neuropathy Management of postherpetic neuralgia Adjunctive therapy for the treatment of partial-onset seizures in patients 17 years of age and older Management of fibromyalgia Management of neuropathic pain associated with spinal cord injury Pediatric use information is approved for Pfizer's LYRICA (pregabalin) Capsules and Oral Solution products. However, due to Pfizer's marketing exclusivity rights, this drug product is not labeled with that pediatric information. Pregabalin capsules are indicated for: Neuropathic pain associated with diabetic peripheral neuropathy (DPN) (1 ) Postherpetic neuralgia (PHN) (1) Adjunctive therapy for the treatment of partial-onset seizures in patients 17 years of age and older ( 1) Fibromyalgia ( 1) Neuropathic pain associated with spinal cord injury (1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION For adult indications, begin dosing at 150 mg/day. ( 2.2 , 2.3 , 2.4 , 2.5 , 2.6 ) Dosing recommendations: INDICATION Dosing Regimen Maximum Dose DPN Pain (2.2) 3 divided doses per day 300 mg/day within 1 week PHN (2.3) 2 or 3 divided doses per day 300 mg/day within 1 week. Maximum dose of 600 mg/day. Adjunctive Therapy for Partial-Onset Seizures in Adult Patients 17 years of Age and Older (2.4) 2 or 3 divided doses per day Maximum dose of 600 mg/day. Fibromyalgia (2.5) 2 divided doses per day 300 mg/day within 1 week. Maximum dose of 450 mg/day. Neuropathic Pain Associated with Spinal Cord Injury (2.6) 2 divided doses per day 300 mg/day within 1 week. Maximum dose of 600 mg/day. Dose should be adjusted in adult patients with reduced renal function. (2.7) 2.1 Important Administration Instructions Pregabalin capsules are given orally with or without food. When discontinuing pregabalin capsules, taper gradually over a minimum of 1 week [see Warnings and Precautions (5.3) ] . Because pregabalin is eliminated primarily by renal excretion, adjust the dose in adult patients with reduced renal function [see Dosage and Administration (2.7) ] . 2.2 Neuropathic Pain Associated with Diabetic Peripheral Neuropathy in Adults The maximum recommended dose of pregabalin capsules is 100 mg three times a day (300 mg/day) in patients with creatinine clearance of at least 60 mL/min. Begin dosing at 50 mg three times a day (150 mg/day). The dose may be increased to 300 mg/day within 1 week based on efficacy and tolerability. Although pregabalin was also studied at 600 mg/day, there is no evidence that this dose confers additional significant benefit and this dose was less well tolerated. In view of the dose-dependent adverse reactions, treatment with doses above 300 mg/day is not recommended [see Adverse Reactions (6.1) ] . 2.3 Postherpetic Neuralgia in Adults The recommended dose of pregabalin capsules is 75 to 150 mg two times a day, or 50 to 100 mg three times a day (150 to 300 mg/day) in patients with creatinine clearance of at least 60 mL/min. Begin dosing at 75 mg two times a day, or 50 mg three times a day (150 mg/day). The dose may be increased to 300 mg/day within 1 week based on efficacy and tolerability. Patients who do not experience sufficient pain relief following 2 to 4 weeks of treatment with 300 mg/day, and who are able to tolerate pregabalin, may be treated with up to 300 mg two times a day, or 200 mg three times a day (600 mg/day). In view of the dose-dependent adverse reactions and the higher rate of treatment discontinuation due to adverse reactions, reserve dosing above 300 mg/day for those patients who have on-going pain and are tolerating 300 mg daily [see Adverse Reactions (6.1) ] . 2.4 Adjunctive Therapy for Partial-Onset Seizures in Patients 17 Years of Age and Older The recommended dosage for adult patients 17 years of age and older is included in Table 1. Administer the total daily dosage orally in two or three divided doses as indicated in Table 1. Based on clinical response and tolerability, dosage may be increased, approximately weekly. Table 1. Recommended Dosage for Adult Patients 17 Years and Older Age and Body Weight Recommended Initial Dosage Recommended Maximum Dosage Frequency of Administration Adults (17 years and older) 150 mg/day 600 mg/day 2 or 3 divided doses Both the efficacy and adverse event profiles of pregabalin have been shown to be dose-related. The effect of dose escalation rate on the tolerability of pregabalin has not been formally studied. The efficacy of adjunctive pregabalin in patients taking gabapentin has not been evaluated in controlled trials. Consequently, dosing recommendations for the use of pregabalin with gabapentin cannot be offered. Pediatric use information is approved for Pfizer's LYRICA (pregabalin) Capsules and Oral Solution products. However, due to Pfizer's marketing exclusivity rights, this drug product is not labeled with that pediatric information. 2.5 Management of Fibromyalgia in Adults The recommended dose of pregabalin capsules for fibromyalgia is 300 to 450 mg/day. Begin dosing at 75 mg two times a day (150 mg/day). The dose may be increased to 150 mg two times a day (300 mg/day) within 1 week based on efficacy and tolerability. Patients who do not experience sufficient benefit with 300 mg/day may be further increased to 225 mg two times a day (450 mg/day). Although pregabalin was also studied at 600 mg/day, there is no evidence that this dose confers additional benefit and this dose was less well tolerated. In view of the dose-dependent adverse reactions, treatment with doses above 450 mg/day is not recommended [see Adverse Reactions (6.1) ] . 2.6 Neuropathic Pain Associated with Spinal Cord Injury in Adults The recommended dose range of pregabalin capsules for the treatment of neuropathic pain associated with spinal cord injury is 150 to 600 mg/day. The recommended starting dose is 75 mg two times a day (150 mg/day). The dose may be increased to 150 mg two times a day (300 mg/day) within 1 week based on efficacy and tolerability. Patients who do not experience sufficient pain relief after 2 to 3 weeks of treatment with 150 mg two times a day and who tolerate pregabalin may be treated with up to 300 mg two times a day [see Clinical Studies (14.5) ] . 2.7 Dosing for Adult Patients with Renal Impairment In view of dose-dependent adverse reactions and since pregabalin is eliminated primarily by renal excretion, adjust the dose in adult patients with reduced renal function. The use of pregabalin capsules in pediatric patients with compromised renal function has not been studied. Base the dose adjustment in patients with renal impairment on creatinine clearance (CLcr), as indicated in Table 2. To use this dosing table, an estimate of the patient's CLcr in mL/min is needed. CLcr in mL/min may be estimated from serum creatinine (mg/dL) determination using the Cockcroft and Gault equation: Next, refer to the Dosage and Administration section to determine the recommended total daily dose based on indication, for a patient with normal renal function (CLcr greater than or equal to 60 mL/min). Then refer to Table 2 to determine the corresponding renal adjusted dose. (For example: A patient initiating pregabalin therapy for postherpetic neuralgia with normal renal function (CLcr greater than or equal to 60 mL/min), receives a total daily dose of 150 mg/day pregabalin. Therefore, a renal impaired patient with a CLcr of 50 mL/min would receive a total daily dose of 75 mg/day pregabalin administered in two or three divided doses.) For patients undergoing hemodialysis, adjust the pregabalin daily dose based on renal function. In addition to the daily dose adjustment, administer a supplemental dose immediately following every 4-hour hemodialysis treatment (see Table 2). Table 2. Pregabalin Dosage Adjustment Based on Renal Function Creatinine Clearance (CLcr) (mL/min) Total Pregabalin Daily Dose (mg/day)* Dose Regimen Greater than or equal to 60 150 300 450 600 BID or TID 30 to 60 75 150 225 300 BID or TID 15 to 30 25 to 50 75 100 to 150 150 QD or BID Less than 15 25 25 to 50 50 to 75 75 QD Supplementary dosage following hemodialysis (mg) † Patients on the 25 mg QD regimen: take one supplemental dose of 25 mg or 50 mg Patients on the 25 to 50 mg QD regimen: take one supplemental dose of 50 mg or 75 mg Patients on the 50 to 75 mg QD regimen: take one supplemental dose of 75 mg or 100 mg Patients on the 75 mg QD regimen: take one supplemental dose of 100 mg or 150 mg TID = Three divided doses; BID = Two divided doses; QD = Single daily dose. * Total daily dose (mg/day) should be divided as indicated by dose regimen to provide mg/dose. † Supplementary dose is a single additional dose. pregabalin-equation
