Drug Catalog - Product Detail
PREDNISONE TB 10MG 10X10 UD
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00054-0017-20 | HIKMA PHARMACEUTICALS USA | 100 | 10MG | TABLET |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
DESCRIPTION PREMARIN ® (conjugated estrogens tablets, USP) for oral administration contains a mixture of conjugated estrogens obtained exclusively from natural sources, occurring as the sodium salts of water-soluble estrogen sulfates blended to represent the average composition of material derived from pregnant mares' urine. It is a mixture of sodium estrone sulfate and sodium equilin sulfate. It contains as concomitant components, as sodium sulfate conjugates, 17α-dihydroequilin, 17α-estradiol, and 17β-dihydroequilin. Tablets for oral administration are available in 0.3 mg, 0.45 mg, 0.625 mg, 0.9 mg, and 1.25 mg strengths of conjugated estrogens. PREMARIN 0.3 mg, 0.45 mg, 0.625 mg, 0.9 mg, and 1.25 mg tablets also contain the following inactive ingredients: calcium phosphate tribasic, carnauba wax, hydroxypropyl cellulose, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, powdered cellulose, sucrose, and titanium dioxide. — 0.3 mg tablets also contain: D&C Yellow No. 10 and FD&C Blue No. 2. — 0.45 mg tablets also contain: FD&C Blue No. 2. — 0.625 mg tablets also contain: FD&C Blue No. 2 and FD&C Red No. 40. — 0.9 mg tablets also contain: D&C Red No. 30 and D&C Red No. 7. — 1.25 mg tablets also contain: black iron oxide, D&C Yellow No. 10 and FD&C Yellow No. 6. PREMARIN tablets comply with USP Dissolution Test criteria as outlined below: PREMARIN 1.25 mg tablets USP Dissolution Test 4 PREMARIN 0.3 mg, 0.45 mg and 0.625 mg tablets USP Dissolution Test 5 PREMARIN 0.9 mg tablets USP Dissolution Test 6
How Supplied
HOW SUPPLIED PREMARIN ® (conjugated estrogens tablets, USP) — Each oval green tablet contains 0.3 mg. — Each oval blue tablet contains 0.45 mg. — Each oval maroon tablet contains 0.625 mg. — Each oval white tablet contains 0.9 mg. — Each oval yellow tablet contains 1.25 mg. The appearance of these tablets is a trademark of Wyeth Pharmaceuticals. They are supplied by State of Florida DOH Central Pharmacy as follows: NDC Strength Quantity/Form Color Source Prod. Code 53808-0770-1 0.625 mg 30 Tablets in a Blister Pack MAROON 0046-1102 Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature]. Dispense in a well-closed container, as defined in the USP.
Indications & Usage
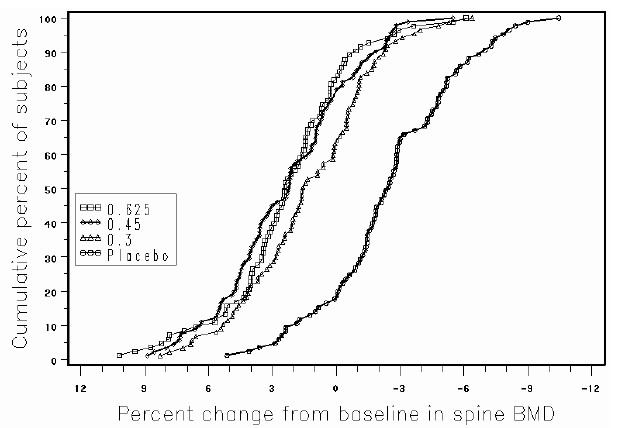
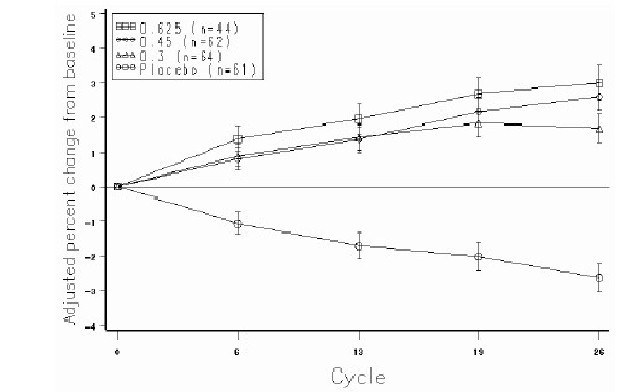
INDICATIONS AND USAGE PREMARIN therapy is indicated in the: Treatment of moderate to severe vasomotor symptoms due to menopause. Treatment of moderate to severe symptoms of vulvar and vaginal atrophy due to menopause. When prescribing solely for the treatment of symptoms of vulvar and vaginal atrophy, topical vaginal products should be considered. Treatment of hypoestrogenism due to hypogonadism, castration or primary ovarian failure. Treatment of breast cancer (for palliation only) in appropriately selected women and men with metastatic disease. Treatment of advanced androgen-dependent carcinoma of the prostate (for palliation only). Prevention of postmenopausal osteoporosis. When prescribing solely for the prevention of postmenopausal osteoporosis, therapy should only be considered for women at significant risk of osteoporosis and for whom non-estrogen medications are not considered to be appropriate. (See CLINICAL STUDIES .) The mainstays for decreasing the risk of postmenopausal osteoporosis are weight-bearing exercise, adequate calcium and vitamin D intake, and when indicated, pharmacologic therapy. Postmenopausal women require an average of 1500 mg/day of elemental calcium. Therefore, when not contraindicated, calcium supplementation may be helpful for women with suboptimal dietary intake. Vitamin D supplementation of 400-800 IU/day may also be required to ensure adequate daily intake in postmenopausal women.
Dosage and Administration
DOSAGE AND ADMINISTRATION When estrogen is prescribed for a postmenopausal woman with a uterus, progestin should also be initiated to reduce the risk of endometrial cancer. A woman without a uterus does not need progestin. Use of estrogen, alone or in combination with a progestin, should be with the lowest effective dose and for the shortest duration consistent with treatment goals and risks for the individual woman. Patients should be reevaluated periodically as clinically appropriate (for example at 3-month to 6-month intervals) to determine if treatment is still necessary (see BOXED WARNINGS and WARNINGS ). For women with a uterus, adequate diagnostic measures, such as endometrial sampling, when indicated, should be undertaken to rule out malignancy in cases of undiagnosed persistent or recurring abnormal vaginal bleeding. PREMARIN may be taken without regard to meals.
