Drug Catalog - Product Detail
PREDNISOLONE ACETATE OPHTH SUSP, USP SUSP 0.01 10ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 61314-0637-10 | SANDOZ | 10 | 1% | SUSPENSION |
PACKAGE FILES

Generic Name
PREDNISOLONE ACETATE
Substance Name
PREDNISOLONE ACETATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
OPHTHALMIC
Application Number
NDA017469
Description
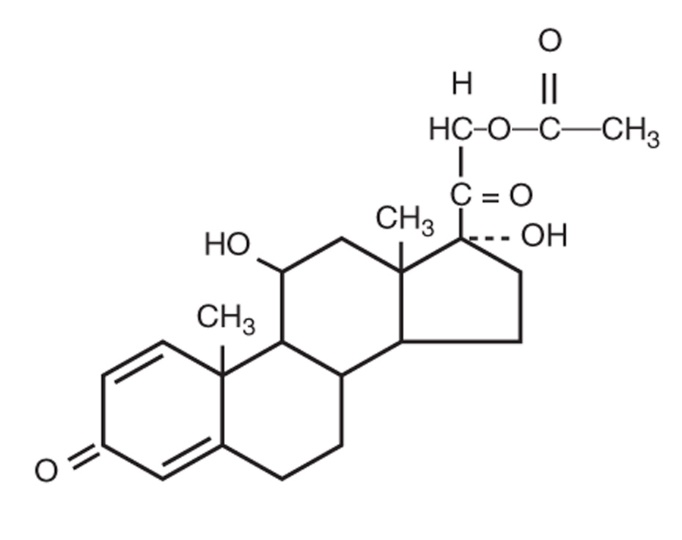
DESCRIPTION: Prednisolone acetate ophthalmic suspension is an adrenocortical steroid product prepared as sterile ophthalmic suspension for topical ophthalmic use. The active ingredient is represented by the chemical structure: Established name: Prednisolone Acetate Chemical name: Pregna-1,4-diene-3,20-dione, 21-(acetyloxy)-11,17-dihydroxy-,(11β)-. Each mL contains: Active : prednisolone acetate 1.0%. Preservative : benzalkonium chloride 0.01%. Inactives: citric acid monohydrate (to adjust pH), dibasic sodium phosphate, edetate disodium, glycerin, hypromellose, polysorbate 80, purified water, sodium hydroxide (to adjust pH). chemical
How Supplied
HOW SUPPLIED: Prednisolone acetate ophthalmic suspension is supplied in a white, round low density polyethylene dispenser with a natural low density polyethylene dispensing plug and pink polypropylene cap. Tamper evidence is provided with a shrink band around the closure and neck area of the package. Prednisolone Acetate Suspension, USP, 1%: 5 mL NDC 61314-637-05 10 mL NDC 61314-637-10 15 mL NDC 61314-637-15 STORAGE: Store at 8°C to 24°C (46°F to 75°F) in an UPRIGHT position. After opening, prednisolone acetate ophthalmic suspension can be used until the expiration date on the bottle. Rx Only Distributed by Sandoz Inc. Princeton, NJ 08540 Rev. Dec 2024
Indications & Usage
INDICATIONS AND USAGE: Steroid responsive inflammatory conditions of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the globe, such as allergic conjunctivitis, acne rosacea, superficial punctate keratitis, herpes zoster keratitis, iritis, cyclitis, selected infective conjunctivitides, when the inherent hazard of steroid use is accepted to obtain an advisable diminution in edema and inflammation; corneal injury from chemical, radiation, or thermal burns, or penetration of foreign bodies.
Dosage and Administration
DOSAGE AND ADMINISTRATION: SHAKE WELL BEFORE USING . Two drops topically in the affected eye(s) four times daily. In cases of bacterial infections, concomitant use of anti-infective agents is mandatory. Care should be taken not to discontinue therapy prematurely. If signs and symptoms fail to improve after two days, the patient should be reevaluated (see PRECAUTIONS ). The dosing of prednisolone acetate ophthalmic suspension may be reduced, but care should be taken not to discontinue therapy prematurely. In chronic conditions, withdrawal of treatment should be carried out by gradually decreasing the frequency of applications.
