Drug Catalog - Product Detail
PRAVASTATIN SODIUM TB 40MG 500
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68462-0197-05 | GLENMARK PHARMACEUTICALS | 500 | 40MG | TABLET |
PACKAGE FILES



Generic Name
Substance Name
Product Type
Route
Application Number
Description
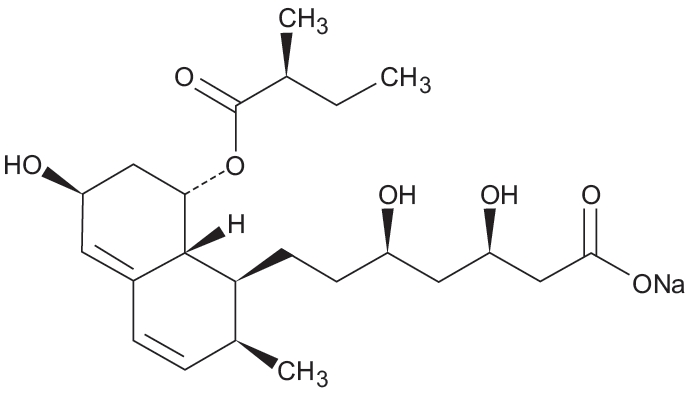
11 DESCRIPTION Pravastatin sodium tablets USP are one of a class of lipid-lowering compounds, the statins, which reduce cholesterol biosynthesis. These agents are competitive inhibitors of HMG-CoA reductase, the enzyme catalyzing the early rate-limiting step in cholesterol biosynthesis, conversion of HMG-CoA to mevalonate. Pravastatin sodium USP is designated chemically as 1-Naphthalene-heptanoic acid, 1,2,6,7,8,8a-hexahydro-β,δ,6-trihydroxy-2-methyl-8-(2-methyl-1-oxobutoxy)-, monosodium salt, [1S[1α(βS*,δS*),2α,6α,8β(R*),8aα]]-. Structural formula: C 23 H 35 NaO 7 MW 446.52 Pravastatin sodium USP is an odorless, white to yellowish white, hygroscpic powder. It is a relatively polar hydrophilic compound with a partition coefficient (octanol/water) of 0.59 at a pH of 7. It is freely soluble in water and methanol, soluble in alcohol, very slightly soluble in acetonitrile and practically insoluble in ether, ethyl acetate and chloroform. Pravastatin sodium tablets USP are available for oral administration as 10 mg, 20 mg, 40 mg, and 80 mg tablets. Inactive ingredients include: colloidal silicon dioxide, crospovidone, hydroxypropyl methylcellulose, magnesium stearate, mannitol, meglumine, microcrystalline cellulose and corn starch. The 10 mg, 20 mg and 80 mg tablets also contain D&C yellow no. 10 aluminum lake and the 40 mg tablet also contains D&C yellow no. 10 aluminum lake and FD&C blue no. 1 aluminum lake. Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied Pravastatin sodium tablets USP are supplied as: 10 mg tablets: Yellow colored, circular shaped tablets having flat surface, with “G5” debossed on one surface and “10” debossed on the other surface. They are supplied in bottles of 90 (NDC 68462-195-90) and bottles of 500 (NDC 68462-195-05). Bottles contain a desiccant canister. 20 mg tablets: Yellow rounded-rectangular tablets having biconvex surface, with “G5” debossed on one surface and “20” debossed on the other surface. They are supplied in bottles of 90 (NDC 68462-196-90) and bottles of 500 (NDC 68462-196-05). Bottles contain a desiccant canister. 40 mg tablets: Green rounded-rectangular tablets having biconvex surface, with “G5” debossed on one surface and “40” debossed on the other surface. They are supplied in bottles of 90 (NDC 68462-197-90) and bottles of 500 (NDC 68462-197-05). Bottles contain a desiccant canister. 80 mg tablets: Yellow oval tablets having biconvex surface, with “G5” debossed on one surface and “80” debossed on the other surface. They are supplied in bottles of 90 (NDC 68462-198-90) and bottles of 500 (NDC 68462-198-05). Bottles contain a desiccant canister. 16.2 Storage Store at 20 o to 25 o C (68 o to 77 o F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]. Keep tightly closed (protect from moisture). Protect from light.
Indications & Usage
1 INDICATIONS AND USAGE Therapy with lipid-altering agents should be only one component of multiple risk factor intervention in individuals at significantly increased risk for atherosclerotic vascular disease due to hypercholesterolemia. Drug therapy is indicated as an adjunct to diet when the response to a diet restricted in saturated fat and cholesterol and other nonpharmacologic measures alone has been inadequate. Pravastatin sodium tablets USP are an HMG-CoA reductase inhibitor (statin) indicated as an adjunctive therapy to diet to: Reduce the risk of MI, revascularization, and cardiovascular mortality in hypercholesterolemic patients without clinically evident CHD. ( 1.1 ) Reduce elevated Total-C, LDL-C, ApoB, and TG levels and to increase HDL-C in patients with primary hypercholesterolemia and mixed dyslipidemia. ( 1.2 ) Reduce elevated serum TG levels in patients with hypertriglyceridemia. ( 1.2 ) Treat patients with primary dysbetalipoproteinemia who are not responding to diet. ( 1.2 ) Treat children and adolescent patients ages 8 years and older with heterozygous familial hypercholesterolemia after failing an adequate trial of diet therapy. ( 1.2 ) Limitations of use: Pravastatin sodium tablets USP have not been studied in Fredrickson Types I and V dyslipidemias. ( 1.3 ) 1.1 Prevention of Cardiovascular Disease In hypercholesterolemic patients without clinically evident coronary heart disease (CHD), pravastatin sodium tablets USP are indicated to: reduce the risk of myocardial infarction (MI). reduce the risk of undergoing myocardial revascularization procedures. reduce the risk of cardiovascular mortality with no increase in death from non-cardiovascular causes. 1.2 Hyperlipidemia Pravastatin sodium tablets USP are indicated: as an adjunct to diet to reduce elevated total cholesterol (Total-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein B (ApoB), and triglyceride (TG) levels and to increase high-density lipoprotein cholesterol (HDL-C) in patients with primary hypercholesterolemia and mixed dyslipidemia ( Fredrickson Types IIa and IIb). 1 as an adjunct to diet for the treatment of patients with elevated serum TG levels (Fredrickson Type IV). for the treatment of patients with primary dysbetalipoproteinemia ( Fredrickson Type III) who do not respond adequately to diet. as an adjunct to diet and lifestyle modification for treatment of heterozygous familial hypercholesterolemia (HeFH) in children and adolescent patients ages 8 years and older if after an adequate trial of diet the following findings are present: LDL-C remains ≥190 mg/dL or LDL-C remains ≥160 mg/dL and: there is a positive family history of premature cardiovascular disease (CVD) or two or more other CVD risk factors are present in the patient. 1.3 Limitations of Use Pravastatin sodium tablets USP have not been studied in conditions where the major lipoprotein abnormality is elevation of chylomicrons (Fredrickson Types I and V).
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Adults: the recommended starting dose is 40 mg once daily. Use 80 mg dose only for patients not reaching LDL-C goal with 40 mg. ( 2.2 ) Significant renal impairment: the recommended starting dose is 10 mg once daily. ( 2.2 ) Children (ages 8 to 13 years, inclusive): the recommended starting dose is 20 mg once daily. ( 2.3 ) Adolescents (ages 14 to 18 years): the recommended starting dose is 40 mg once daily. ( 2.3 ) 2.1 General Dosing Information The patient should be placed on a standard cholesterol-lowering diet before receiving pravastatin sodium tablets and should continue on this diet during treatment with pravastatin sodium tablets [see NCEP Treatment Guidelines for details on dietary therapy]. 2.2 Adult Patients The recommended starting dose is 40 mg once daily. If a daily dose of 40 mg does not achieve desired cholesterol levels, 80 mg once daily is recommended. In patients with significant renal impairment, a starting dose of 10 mg daily is recommended. Pravastatin sodium tablets can be administered orally as a single dose at any time of the day, with or without food. Since the maximal effect of a given dose is seen within 4 weeks, periodic lipid determinations should be performed at this time and dosage adjusted according to the patient’s response to therapy and established treatment guidelines. 2.3 Pediatric Patients Children (Ages 8 to 13 Years, Inclusive) The recommended dose is 20 mg once daily in children 8 to 13 years of age. Doses greater than 20 mg have not been studied in this patient population. Adolescents (Ages 14 to 18 Years) The recommended starting dose is 40 mg once daily in adolescents 14 to 18 years of age. Doses greater than 40 mg have not been studied in this patient population. Children and adolescents treated with pravastatin should be reevaluated in adulthood and appropriate changes made to their cholesterol-lowering regimen to achieve adult goals for LDL-C [see Indications and Usage ( 1.2 ) ]. 2.4 Concomitant Lipid-Altering Therapy Pravastatin sodium tablets may be used with bile acid resins. When administering a bile-acid-binding resin (e.g., cholestyramine, colestipol) and pravastatin, pravastatin sodium tablets should be given either 1 hour or more before or at least 4 hours following the resin. [See Clinical Pharmacology ( 12.3 ) .] 2.5 Dosage in Patients Taking Cyclosporine In patients taking immunosuppressive drugs such as cyclosporine concomitantly with pravastatin, therapy should begin with 10 mg of pravastatin sodium once-a-day at bedtime and titration to higher doses should be done with caution. Most patients treated with this combination received a maximum pravastatin sodium dose of 20 mg/day. In patients taking cyclosporine, therapy should be limited to 20 mg of pravastatin sodium once daily [see Warnings and Precautions ( 5.1 ) and Drug Interactions ( 7.1 ) ]. 2.6 Dosage in Patients Taking Clarithromycin In patients taking clarithromycin, therapy should be limited to 40 mg of pravastatin sodium once daily [see Drug Interactions ( 7.2 ) ].
