Drug Catalog - Product Detail
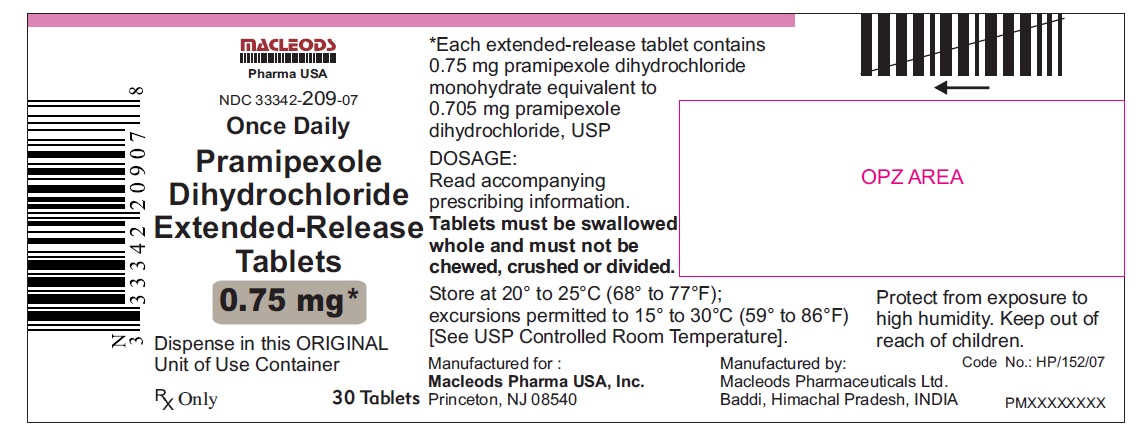
PRAMIPEXOLE DIHYDROCHLORIDE TAB ER 24HR 0.75 MG 30 CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 33342-0209-07 | MACLEODS PHARMACEUTICALS | 30 | 0.75MG | NA |
PACKAGE FILES








Generic Name
PRAMIPEXOLE DIHYDROCHLORIDE
Substance Name
PRAMIPEXOLE DIHYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA206156
Description
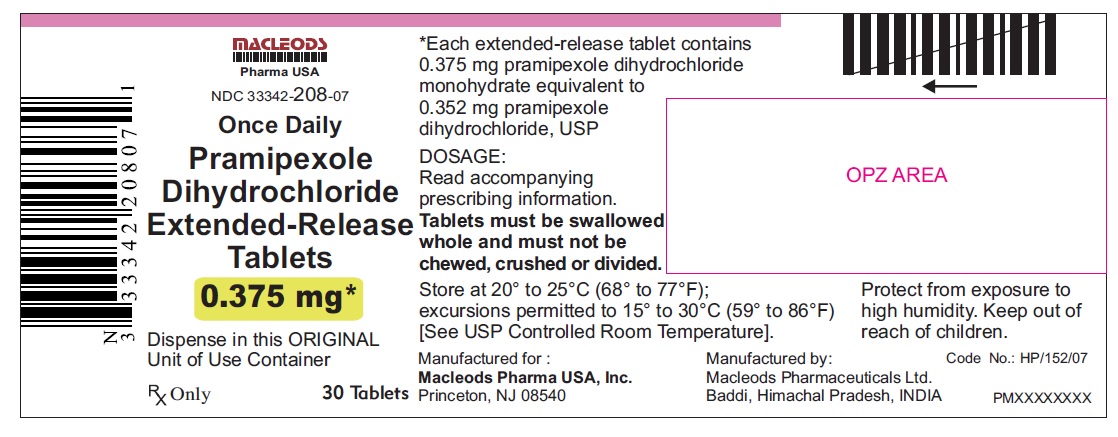
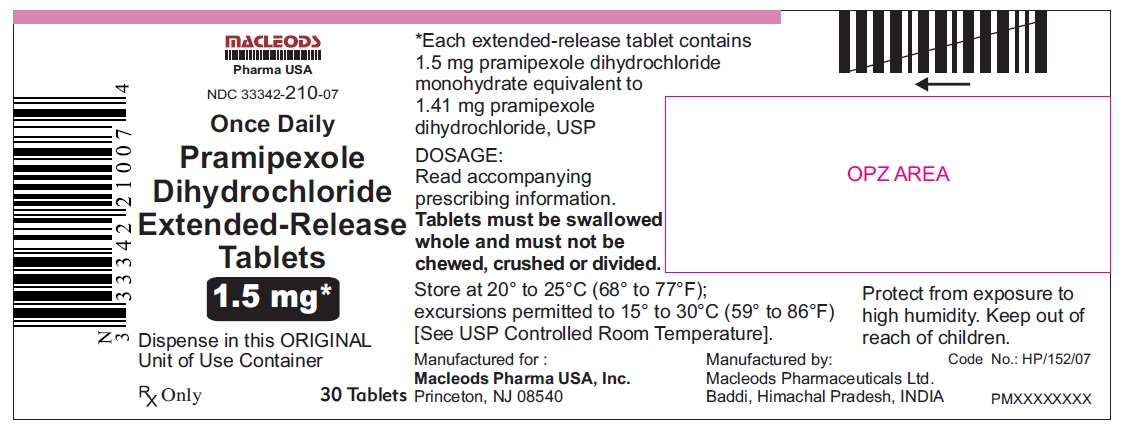
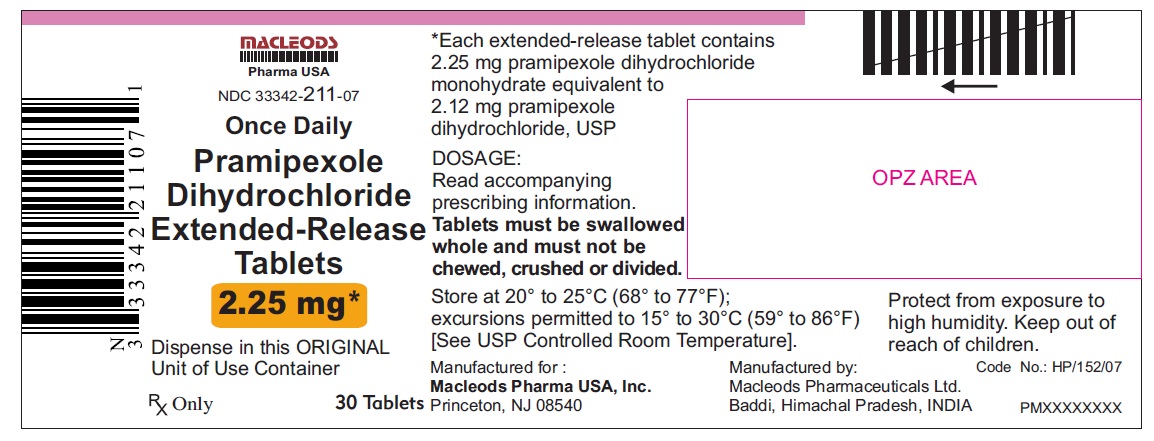
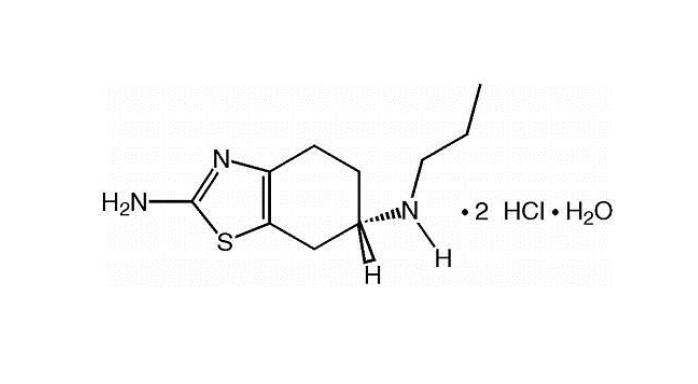
11 DESCRIPTION Pramipexole dihydrochloride extended-release tablets contain pramipexole dihydrochloride (as a monohydrate). Pramipexole is a non-ergot dopamine agonist. The chemical name of pramipexole dihydrochloride monohydrate is ( S )-2-amino-4,5,6,7-tetrahydro-6-(propylamino) benzothiazole dihydrochloride monohydrate. Its empirical formula is C 10 H 17 N 3 S · 2HCl · H 2 O, and its molecular weight is 302.26. The structural formula is: Pramipexole dihydrochloride USP is a white to off-white powder substance. Melting occurs in the range of 296°C to 301°C, with decomposition. Pramipexole dihydrochloride USP is more than 20% soluble in water, about 8% in methanol, about 0.5% in ethanol, and practically insoluble in dichloromethane. Pramipexole dihydrochloride extended-release tablets, 0.375 mg: Each extended-release tablet contains 0.375 mg pramipexole dihydrochloride monohydrate equivalent to 0.352 mg pramipexole dihydrochloride USP. Pramipexole dihydrochloride extended-release tablets, 0.75 mg: Each extended-release tablet contains 0.75 mg pramipexole dihydrochloride monohydrate equivalent to 0.705 mg pramipexole dihydrochloride USP. Pramipexole dihydrochloride extended-release tablets, 1.5 mg: Each extended-release tablet contains 1.5 mg pramipexole dihydrochloride monohydrate equivalent to 1.41 mg pramipexole dihydrochloride USP. Pramipexole dihydrochloride extended-release tablets, 2.25 mg: Each extended-release tablet contains 2.25 mg pramipexole dihydrochloride monohydrate equivalent to 2.12 mg pramipexole dihydrochloride USP. Pramipexole dihydrochloride extended-release tablets, 3 mg: Each extended-release tablet contains 3 mg pramipexole dihydrochloride monohydrate equivalent to 2.82 mg pramipexole dihydrochloride USP. Pramipexole dihydrochloride extended-release tablets, 3.75 mg: Each extended-release tablet contains 3.75 mg pramipexole dihydrochloride monohydrate equivalent to 3.53 mg pramipexole dihydrochloride USP. Pramipexole dihydrochloride extended-release tablets, 4.5 mg: Each extended-release tablet contains 4.5 mg pramipexole dihydrochloride monohydrate equivalent to 4.23 mg pramipexole dihydrochloride USP. Inactive ingredients for all strengths of pramipexole dihydrochloride extended-release tablets consist of hypromellose, mannitol, pregelatinized starch, carbomer homopolymer, colloidal silicon dioxide, and magnesium stearate. prami
How Supplied
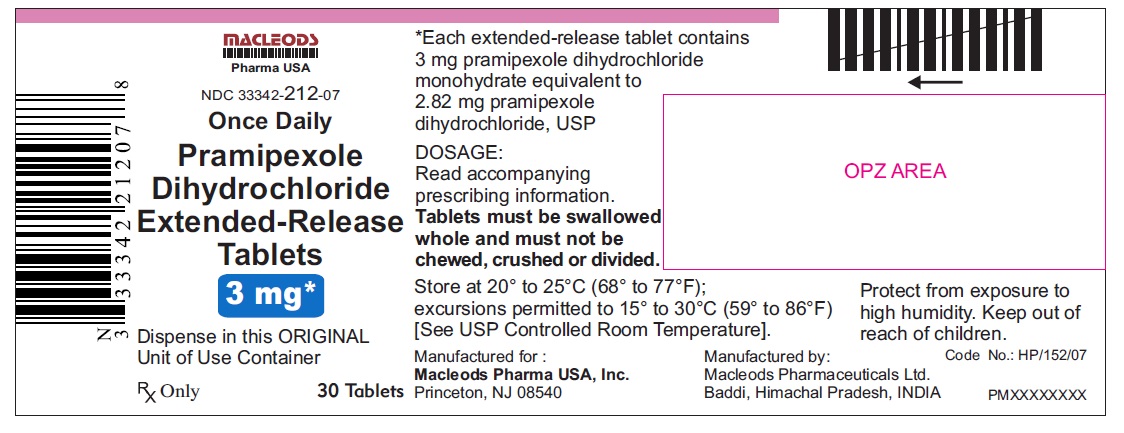
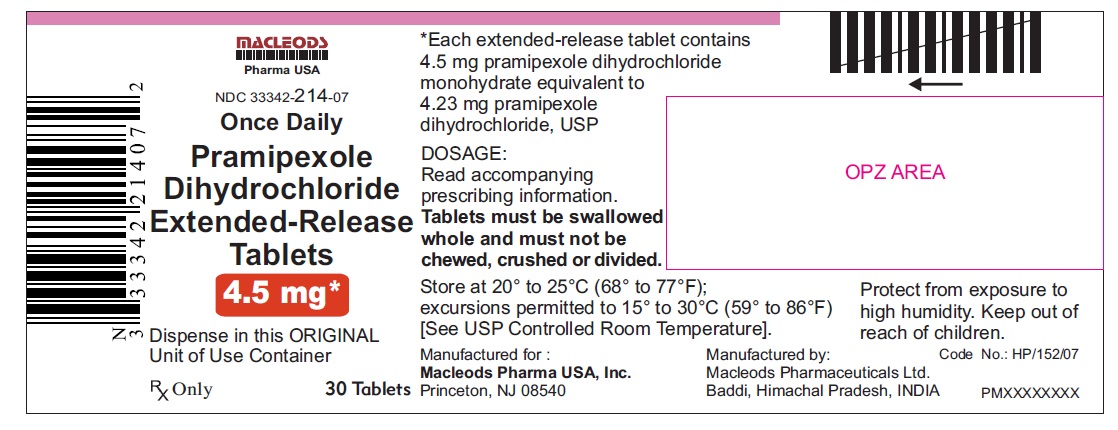
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied Pramipexole dihydrochloride extended-release tablets are available as follows: 0. 375 mg: White colored, circular, flat, beveled edged, uncoated tablet debossed ‘ER 1’ on one side of the tablet and ‘0.375’ on other side. Blister pack of 100(10 x 10) tablets NDC 33342-208-12 Unit of Use Bottles of 30 NDC 33342-208-07 0.75 mg: White colored, circular, flat, beveled edged, uncoated tablet debossed ‘ER 2’ on one side of the tablet and ‘0.75’ on other side. Blister pack of 100(10 x 10) tablets NDC 33342-209-12 Unit of Use Bottles of 30 NDC 33342-209-07 1.5 mg: White colored, oval shaped, biconvex, uncoated tablet debossed ‘ER 3’ on one side of the tablet and ‘1.5’ on other side. Blister pack of 100(10 x 10) tablets NDC 33342-210-12 Unit of Use Bottles of 30 NDC 33342-210-07 2.25 mg: White colored, oval shaped, biconvex, uncoated tablet debossed ‘ER 4’ on one side of the tablet and ‘2.25’ on other side. Blister pack of 100(10 x 10) tablets NDC 33342-211-12 Unit of Use Bottles of 30 NDC 33342-211-07 3.0 mg: White colored, oval shaped, biconvex, uncoated tablet debossed ‘ER 5’ on one side of the tablet and ‘3.0’ on other side. Blister pack of 100(10 x 10) tablets NDC 33342-212-12 Unit of Use Bottles of 30 NDC 33342-212-07 3.75 mg: White colored, oval shaped, biconvex, uncoated tablet debossed ‘ER 6’ on one side of the tablet and ‘3.75’ on other side. Blister pack of 100(10 x 10) tablets NDC 33342-213-12 Unit of Use Bottles of 30 NDC 33342-213-07 4.5 mg: White colored, oval shaped, biconvex, uncoated tablet debossed ‘ER 7’ on one side of the tablet and ‘4.5’ on other side. Blister pack of 100(10 x 10) tablets NDC 33342-214-12 Unit of Use Bottles of 30 NDC 33342-214-07 16.2 Storage and Handling Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Protect from exposure to high humidity. Store in a safe place out of the reach of children.
Indications & Usage
1 INDICATIONS AND USAGE Pramipexole dihydrochloride extended-release tablets are indicated for the treatment of Parkinson's disease. Pramipexole dihydrochloride extended-release tablets are a non-ergot dopamine agonist indicated for the treatment of Parkinson's disease (PD) ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION • Pramipexole dihydrochloride extended-release tablets are taken once daily, with or without food ( 2.1 ) • Tablets must be swallowed whole and must not be chewed, crushed, or divided ( 2.1 ) • Starting dose is 0.375 mg given once daily ( 2.2 ) • Dose may be increased gradually, not more frequently than every 5 to 7 days, first to 0.75 mg per day and then by 0.75 mg increments up to a maximum recommended dose of 4.5 mg per day. Assess therapeutic response and tolerability at a minimal interval of 5 days or longer after each dose increment ( 2.2 ) • Patients may be switched overnight from immediate-release pramipexole tablets to pramipexole dihydrochloride extended-release tablets at the same daily dose. Dose adjustment may be needed in some patients ( 2.3 ) • Pramipexole dihydrochloride extended-release tablets should be discontinued gradually ( 2.2 ) 2.1 General Dosing Considerations Pramipexole dihydrochloride extended-release tablets are taken orally once daily, with or without food. Pramipexole dihydrochloride extended-release tablets must be swallowed whole and must not be chewed, crushed, or divided. If a significant interruption in therapy with pramipexole dihydrochloride extended-release tablets has occurred, re-titration of therapy may be warranted. 2.2 Dosing for Parkinson's Disease The starting dose is 0.375 mg given once per day. Based on efficacy and tolerability, dosages may be increased gradually, not more frequently than every 5 to 7 days, first to 0.75 mg per day and then by 0.75 mg increments up to a maximum recommended dose of 4.5 mg per day. In clinical trials, dosage was initiated at 0.375 mg/day and gradually titrated based on individual therapeutic response and tolerability. Doses greater than 4.5 mg/day have not been studied in clinical trials. Patients should be assessed for therapeutic response and tolerability at a minimal interval of 5 days or longer after each dose increment [see Clinical Studies ( 14 )]. Due to the flexible dose design used in clinical trials, specific dose-response information could not be determined [see Clinical Studies ( 14 )]. Pramipexole dihydrochloride extended-release tablets may be tapered off at a rate of 0.75 mg per day until the daily dose has been reduced to 0.75 mg. Thereafter, the dose may be reduced by 0.375 mg per day [ see Warnings and Precautions ( 5.10 , 5.11 )] Dosing in Patients with Renal Impairment In patients with moderate renal impairment (creatinine clearance between 30 and 50 mL/min), pramipexole dihydrochloride extended-release tablets should initially be taken every other day. Caution should be exercised and careful assessment of therapeutic response and tolerability should be made before increasing to daily dosing after one week, and before any additional titration in 0.375 mg increments up to 2.25 mg per day. Dose adjustment should occur no more frequently than at weekly intervals. Pramipexole dihydrochloride extended-release tablets have not been studied in patients with severe renal impairment (creatinine clearance <30 mL/min) or patients on hemodialysis, and are not recommended in these patients. 2.3 Switching from Immediate-Release Pramipexole Tablets to Pramipexole Dihydrochloride Extended-Release Tablets Patients with Parkinson's disease may be switched overnight from immediate-release pramipexole tablets to pramipexole dihydrochloride extended-release tablets at the same daily dose. When switching between immediate-release pramipexole tablets and pramipexole dihydrochloride extended-release tablets, patients should be monitored to determine if dosage adjustment is necessary.
