Drug Catalog - Product Detail
PRAMIPEXOLE DIHYDROCHLORIDE TAB 0.125 MG 90 CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 57237-0180-90 | RISING PHARMACEUTICALS | 90 | 0.125MG | TABLET |
PACKAGE FILES

Generic Name
PRAMIPEXOLE DIHYDROCHLORIDE
Substance Name
PRAMIPEXOLE DIHYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA202633
Description
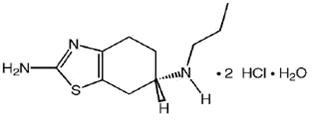
11 DESCRIPTION Pramipexole dihydrochloride tablets contain pramipexole dihydrochloride (as a monohydrate). Pramipexole is a nonergot dopamine agonist. The chemical name of pramipexole dihydrochloride monohydrate is ( S )-2-amino-4,5,6,7-tetrahydro-6-(propylamino)benzothiazole dihydrochloride monohydrate. Its molecular formula is C 10 H 17 N 3 S·2HCl·H 2 O, and its molecular weight is 302.26. The structural formula is: Pramipexole dihydrochloride USP is a white or almost white, crystalline powder. Melting occurs in the range of 296°C to 301°C, with decomposition. Pramipexole dihydrochloride is more than 20% soluble in water, about 8% in methanol, about 0.5% in ethanol, and practically insoluble in dichloromethane. Pramipexole dihydrochloride tablets 0.125 mg: Each tablet contains 0.125 mg pramipexole dihydrochloride monohydrate equivalent to 0.118 mg pramipexole dihydrochloride USP. Pramipexole dihydrochloride tablets 0.25 mg: Each tablet contains 0.25 mg pramipexole dihydrochloride monohydrate equivalent to 0.235 mg pramipexole dihydrochloride USP. Pramipexole dihydrochloride tablets 0.5 mg: Each tablet contains 0.5 mg pramipexole dihydrochloride monohydrate equivalent to 0.47 mg pramipexole dihydrochloride USP. Pramipexole dihydrochloride tablets 0.75 mg: Each tablet contains 0.75 mg pramipexole dihydrochloride monohydrate equivalent to 0.705 mg pramipexole dihydrochloride USP. Pramipexole dihydrochloride tablets 1 mg: Each tablet contains 1 mg pramipexole dihydrochloride monohydrate equivalent to 0.94 mg pramipexole dihydrochloride USP. Pramipexole dihydrochloride tablets 1.5 mg: Each tablet contains 1.5 mg pramipexole dihydrochloride monohydrate equivalent to 1.41 mg pramipexole dihydrochloride USP. Inactive ingredients consist of colloidal silicon dioxide, corn starch, magnesium stearate, mannitol, and povidone. Chemical Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied Pramipexole Dihydrochloride tablets are available as follows: Pramipexole Dihydrochloride Tablets, 0.125 mg are white to off-white, round, flat, beveled edge uncoated tablets, debossed with ‘Y’ on one side ‘41’ on other side. Bottles of 90 NDC 57237-180-90 Bottles of 1,000 NDC 57237-180-99 Pramipexole Dihydrochloride Tablets, 0.25 mg are white to off-white, oval, biconcave, beveled edge uncoated tablets, debossed with ‘Y’ and ‘42’ separated by score line on one side and plain with score line on other side. Bottles of 90 NDC 57237-181-90 Bottles of 1,000 NDC 57237-181-99 Pramipexole Dihydrochloride Tablets, 0.5 mg are white to off-white, oval, biconcave, beveled edge uncoated tablets, debossed with ‘Y’ and ‘43’ separated by score line on one side and plain with score line on other side. Bottles of 90 NDC 57237-182-90 Bottles of 1,000 NDC 57237-182-99 Pramipexole Dihydrochloride Tablets, 0.75 mg are white to off-white, oval, biconcave, beveled edge uncoated tablets, debossed with ‘Y’ on one side and ‘44’ on other side. Bottles of 90 NDC 57237-183-90 Bottles of 1,000 NDC 57237-183-99 Pramipexole Dihydrochloride Tablets, 1 mg are white to off-white, round, flat, beveled edge uncoated tablets, debossed with ‘Y’ and ‘45’ separated by score line on one side and plain with score line on other side. Bottles of 90 NDC 57237-184-90 Bottles of 1,000 NDC 57237-184-99 Pramipexole Dihydrochloride Tablets, 1.5 mg are white to off-white, round, flat, beveled edge uncoated tablets, debossed with ‘Y’ and ‘46’ separated by score line on one side and plain with score line on other side. Bottles of 90 NDC 57237-185-90 Bottles of 1,000 NDC 57237-185-99 16.2 Storage and Handling Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from light. Store in a safe place out of the reach of children.
Indications & Usage
1 INDICATIONS AND USAGE Pramipexole dihydrochloride is a non-ergot dopamine agonist indicated for the treatment of: Parkinson’s disease (PD) ( 1.1 ) Moderate-to-severe primary Restless Legs Syndrome (RLS) (1.2) 1.1 Parkinson's Disease Pramipexole dihydrochloride tablets are indicated for the treatment of Parkinson's disease. 1.2 Restless Legs Syndrome Pramipexole dihydrochloride tablets are indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS).
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Parkinson’s Disease-Normal Renal Function* (2.2) Week Dosage (mg) Total Daily Dose (mg) 1 0.125 TID 0.375 2 0.25 TID 0.75 3 0.5 TID 1.5 4 0.75 TID 2.25 5 1 TID 3 6 1.25 TID 3.75 7 1.5 TID 4.5 * Doses should not be increased more frequently than every 5 to 7 days. Titrate to effective dose. If used with levodopa, may need to reduce levodopa dose. Parkinson’s Disease-Impaired Renal Function (2.2) Creatinine Clearance Starting Dose (mg) Maximum Dose (mg) <15 mL/min and hemodialysis patients Data not available >50 mL/min 0.125 TID 1.5 TID 30 to 50 mL/min 0.125 BID 0.75 TID 15 to 30 mL/min 0.125 QD 1.5 QD Restless Legs Syndrome* (2.3) Titration Step Dose (mg) 2 to 3 hours before bedtime 1 0.125 2 (if needed) 0.25 3 (if needed) 0.5 * Dosing interval is 4 to 7 days (14 days in patients with CrCl 20 to 60 mL/min) 2.1 General Dosing Considerations Pramipexole dihydrochloride tablets are taken orally, with or without food. If a significant interruption in therapy with pramipexole dihydrochloride tablets has occurred, re-titration of therapy may be warranted. 2.2 Dosing for Parkinson's Disease In all clinical studies, dosage was initiated at a subtherapeutic level to avoid intolerable adverse effects and orthostatic hypotension. Pramipexole dihydrochloride tablets should be titrated gradually in all patients. The dose should be increased to achieve a maximum therapeutic effect, balanced against the principal side effects of dyskinesia, hallucinations, somnolence, and dry mouth. Dosing in Patients with Normal Renal Function Initial Treatment Doses should be increased gradually from a starting dose of 0.375 mg/day given in three divided doses and should not be increased more frequently than every 5 to 7 days. A suggested ascending dosage schedule that was used in clinical studies is shown in Table 1: Table 1 Ascending Dosage Schedule of Pramipexole Dihydrochloride tablets for Parkinson's Disease Week Dosage (mg) Total Daily Dose (mg) 1 0.125 three times a day 0.375 2 0.25 three times a day 0.75 3 0.5 three times a day 1.5 4 0.75 three times a day 2.25 5 1 three times a day 3 6 1.25 three times a day 3.75 7 1.5 three times a day 4.5 Maintenance Treatment Pramipexole dihydrochloride tablets were effective and well tolerated over a dosage range of 1.5 to 4.5 mg/day administered in equally divided doses three times per day with or without concomitant levodopa (approximately 800 mg/day). In a fixed-dose study in early Parkinson's disease patients, doses of 3 mg, 4.5 mg, and 6 mg per day of pramipexole dihydrochloride tablets were not shown to provide any significant benefit beyond that achieved at a daily dose of 1.5 mg/day. However, in the same fixed-dose study, the following adverse events were dose related: postural hypotension, nausea, constipation, somnolence, and amnesia. The frequency of these events was generally 2-fold greater than placebo for pramipexole doses greater than 3 mg/day. The incidence of somnolence reported with pramipexole at a dose of 1.5 mg/day was comparable to placebo. When pramipexole dihydrochloride tablets are used in combination with levodopa, a reduction of the levodopa dosage should be considered. In a controlled study in advanced Parkinson's disease, the dosage of levodopa was reduced by an average of 27% from baseline. Dosing in Patients with Renal Impairment The recommended dosing of pramipexole dihydrochloride tablets in Parkinson's disease patients with renal impairment is provided in Table 2. Table 2 Dosing of Pramipexole Dihydrochloride tablets in Parkinson’s Disease Patients with Renal Impairment Renal Status Starting Dose (mg) Maximum Dose (mg) Normal to mild impairment (creatinine Cl >50 mL/min) 0.125 three times a day 1.5 three times a day Moderate impairment (creatinine Cl = 30 to 50 mL/min) 0.125 twice a day 0.75 three times a day Severe impairment (creatinine Cl = 15 to <30 mL/min) 0.125 once a day 1.5 once a day Very severe impairment (creatinine Cl <15 mL/min and hemodialysis patients) The use of pramipexole dihydrochloride tablets has not been adequately studied in this group of patients. Discontinuation of Treatment Pramipexole dihydrochloride tablets may be tapered off at a rate of 0.75 mg per day until the daily dose has been reduced to 0.75 mg. Thereafter, the dose may be reduced by 0.375 mg per day [ see Warnings and Precautions (5.10, 5.11 ) ]. 2.3 Dosing for Restless Legs Syndrome The recommended starting dose of pramipexole dihydrochloride tablets is 0.125 mg taken once daily 2 to 3 hours before bedtime. For patients requiring additional symptomatic relief, the dose may be increased every 4 to 7 days (Table 3). Although the dose of pramipexole dihydrochloride tablets was increased to 0.75 mg in some patients during long-term open-label treatment, there is no evidence that the 0.75 mg dose provides additional benefit beyond the 0.5 mg dose. Table 3 Ascending Dosage Schedule of Pramipexole Dihydrochloride tablets for RLS *if needed Titration Step Duration Dose (mg) to be taken once daily, 2 to 3 hours before bedtime 1 4 to 7 days 0.125 2* 4 to 7 days 0.25 3* 4 to 7 days 0.5 Dosing in Patients with Renal Impairment The duration between titration steps should be increased to 14 days in RLS patients with moderate and severe renal impairment (creatinine clearance 20 to 60 mL/min) [ see Clinical Pharmacology (12.3) ] . Discontinuation of Treatment In clinical trials of patients being treated for RLS with doses up to 0.75 mg once daily, pramipexole dihydrochloride tablets were discontinued without a taper. In a 26 week placebo-controlled clinical trial, patients reported a worsening of RLS symptom severity as compared to their untreated baseline when pramipexole dihydrochloride tablets treatment was suddenly withdrawn [ see Warnings and Precautions (5.10) ].
