Drug Catalog - Product Detail
POTASSIUM CHLORIDE ER CAPS 10MEQ 500CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68180-0799-02 | LUPIN PHARMACEUTICALS | 500 | 10MEQ | CAPSULE |
PACKAGE FILES
Generic Name
POTASSIUM CHLORIDE
Substance Name
POTASSIUM CHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA203002
Description
11 DESCRIPTION Potassium chloride extended-release capsules USP, 8 mEq and 10 mEq are oral dosage forms of microencapsulated potassium chloride containing 600 and 750 mg, respectively, of potassium chloride USP equivalent to 8 and 10 mEq of potassium. The chemical name of the active ingredient is potassium chloride and the structural formula is KCl. It has a molecular mass of 74.55. Potassium chloride, USP, occurs as a white, granular powder or as colorless crystals. It is odorless and has a saline taste. Its solutions are neutral to litmus. It is freely soluble in water and insoluble in alcohol. The inactive ingredients are ethyl cellulose, triethyl citrate, talc, sodium lauryl sulfate, gelatin, titanium dioxide, shellac, propylene glycol, potassium hydroxide. In addition 600 mg [equivalent to 8 mEq of potassium] capsule also contain black iron oxide and 750 mg [equivalent to 10 mEq of potassium] capsule also contain FD&C blue #1 and FD&C red # 40. FDA approved dissolution test specifications differ from USP.
How Supplied
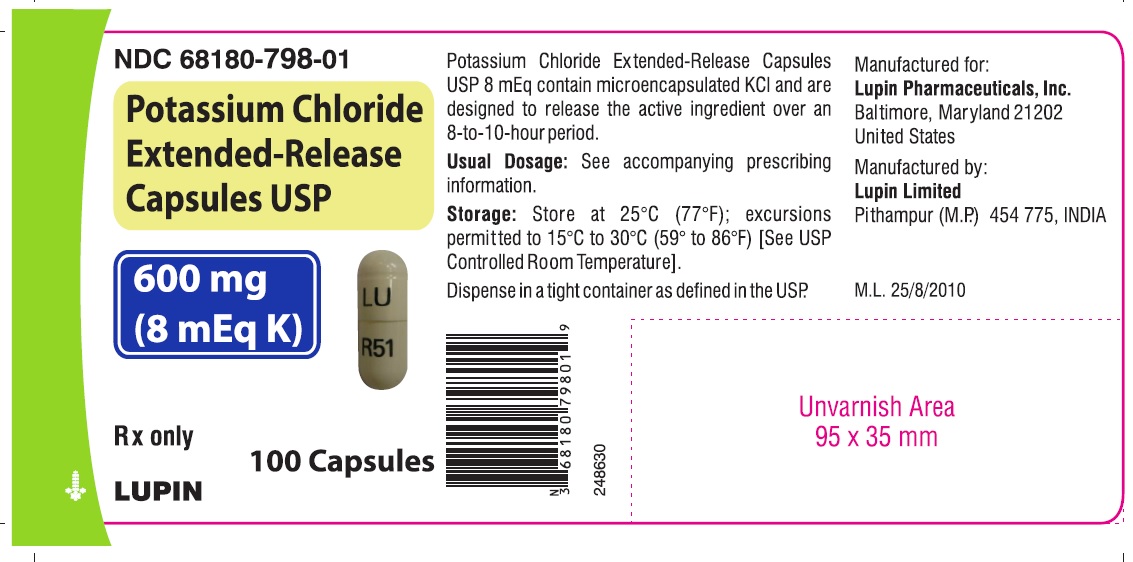
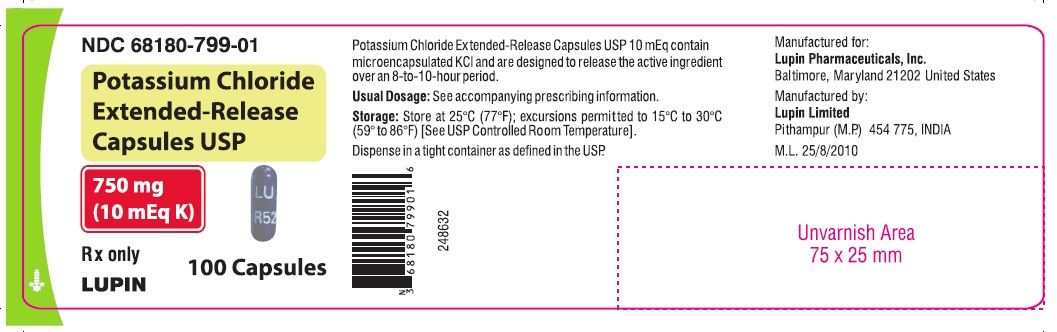
16 HOW SUPPLIED/STORAGE AND HANDLING Potassium chloride Extended-Release Capsules USP contain 600 mg and 750 mg of potassium chloride (equivalent to 8 mEq and 10 mEq of potassium, respectively). Table 1: How Supplied Dose Color Printing NDC Bottle Count 100 500 600 mg (equivalent to 8 mEq of potassium) opaque white "R51" - body 68180-798-01 68180-798-02 - "LU" - cap 100 500 1000 750 mg (equivalent to 10 mEq of potassium) opaque blue "R52" - body 68180-799-01 68180-799-02 68180-799-03 "LU" - cap Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59° to 86°F) [See USP Controlled Room Temperature] . Dispense in tight, light-resistant container as defined in the USP, with a child-resistant closure. Rx only
Indications & Usage
1 INDICATIONS AND USAGE Potassium chloride extended-release capsules are indicated for the treatment and prophylaxis of hypokalemia in adults and children with or without metabolic alkalosis, in patients for whom dietary management with potassium-rich foods or diuretic dose reduction is insufficient. Potassium chloride extended-release capsules, USP contain potassium chloride, a potassium salt indicated for the treatment and prophylaxis of hypokalemia with or without metabolic alkalosis, in patients for whom dietary management with potassium-rich foods or diuretic dose reduction is insufficient.( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Monitor serum potassium and adjust dosage accordingly ( 2.1 ) If serum potassium concentration is <2.5 mEq/L, use intravenous potassium instead of oral supplementation.( 2.1 ) Treatment of hypokalemia : Adults: Typical doses range from 40 to 100 mEq/day in 2 to 5 divided doses; limit doses to 40 mEq per dose. (2.2) Pediatric patients: 2 to 4 mEq/kg/day in divided doses not to exceed 1 mEq/kg as a single dose or 20 mEq, whichever is lower; if deficits are severe or ongoing losses are great, consider intravenous therapy.( 2.3 ) Maintenance or Prophylaxis of hypokalemia : Adults: Typical dose is 20 mEq per day ( 2.2 ) Pediatric patients: Typical dose is 1 mEq/kg/day.( 2.3 ) 2.1 Administration and Monitoring If serum potassium concentration is <2.5 mEq/L, use intravenous potassium instead of oral supplementation. Monitoring Monitor serum potassium and adjust dosages accordingly. Monitor serum potassium periodically during maintenance therapy to ensure potassium remains in desired range. The treatment of potassium depletion, particularly in the presence of cardiac disease, renal disease, or acidosis requires careful attention to acid-base balance, volume status, electrolytes, including magnesium, sodium, chloride, phosphate, and calcium, electrocardiograms and the clinical status of the patient. Correct volume status, acid-base balance and electrolyte deficits as appropriate. Administration Take with meals and with a full glass of water or other liquid. Do not take on an empty stomach because of the potential for gastric irritation [see Warnings and Precautions ( 5.1 )]. Patients who have difficulty swallowing capsules may sprinkle the contents of the capsule onto a spoonful of soft food. The soft food, such as applesauce or pudding, should be swallowed immediately without chewing and followed with a glass of water or juice to ensure complete swallowing of the microcapsules. Do not added to hot foods. Any microcapsule/food mixture should be used immediately and not stored for future use. 2.2 Adult Dosing Dosage must be adjusted to the individual needs of each patient. Dosages greater than 40 mEq per day should be divided such that no more than 40 mEq is given in a single dose. Treatment of hypokalemia: Typical dose range is 40 to 100 mEq per day. Maintenance or Prophylaxis: Typical dose is 20 mEq per day. 2.3 Pediatric Dosing Pediatric patients aged birth to 16 years old: Dosage must be adjusted to the individual needs of each patient. Do not exceed as a single dose 1 mEq/kg or 20 mEq, whichever is lower. Treatment of hypokalemia: The recommended initial dose is 2 to 4 mEq/kg/day in divided doses. If deficits are severe or ongoing losses are great, consider intravenous therapy. Maintenance or Prophylaxis: Typical dose is 1 mEq/kg/day.
