Drug Catalog - Product Detail
PILOCARPINE HCL OPHTH SOLN 1% 15 ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 17478-0223-12 | AKORN | 15 | 1% | NA |
PACKAGE FILES



Generic Name
Substance Name
Product Type
Route
Application Number
Description
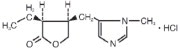
11 DESCRIPTION Pilocarpine Hydrochloride Ophthalmic Solution, USP is a cholinergic agonist prepared as a sterile topical ophthalmic solution. The active ingredient is represented by the chemical structure: Established name: Pilocarpine hydrochloride Chemical name: 2(3 H )-furanone, 3-ethyldihydro-4-[(1-methyl-1 H -imidazol-5-yl)-methyl]-monohydrochloride, (3S- cis )-. Molecular Formula: C 11 H 16 N 2 O 2 • HCl Molecular Weight: 244.72 Each mL of Pilocarpine Hydrochloride Ophthalmic Solution, USP contains: Active: Pilocarpine hydrochloride 1% (10 mg/mL), 2% (20 mg/mL), or 4% (40 mg/mL). Preservative: Benzalkonium chloride 0.01%. Inactives: Hypromellose (2910) 5 mg (0.5%), boric acid, sodium citrate, sodium chloride (present in 1% only); hydrochloric acid and/or sodium hydroxide (to adjust pH); water for injection. Pilocarpine Hydrochloride Ophthalmic Solution, USP has a pH of 3.5 to 5.5 and an osmolality of 270 to 350 mOsm/kg (1% product), 290 to 350 mOsm/kg (2% product) and 500 to 600 mOsm/kg (4% product). structural formula
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Pilocarpine Hydrochloride Ophthalmic Solution, USP 1%, 2% and 4% is supplied sterile in white low density polyethylene plastic ophthalmic bottles and natural low density polyethylene tips with green polypropylene caps. 15 mL in 15 mL bottles 1% - NDC 17478-223-12 2% - NDC 17478-224-12 4% - NDC 17478-226-12 STORAGE: Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from freezing. Keep tightly closed.
Indications & Usage
1 INDICATIONS AND USAGE Pilocarpine hydrochloride ophthalmic solution is indicated for the: Pilocarpine hydrochloride ophthalmic solution is a muscarinic cholinergic agonist indicated for The reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension ( 1.1 ) The management of acute angle-closure glaucoma ( 1.2 ) The prevention of postoperative elevated IOP associated with laser surgery ( 1.3 ) The induction of miosis ( 1.4 ) 1.1 Reduction of Elevated Intraocular Pressure (IOP) in Patients with Open-Angle Glaucoma or Ocular Hypertension 1.2 Management of Acute Angle-Closure Glaucoma 1.3 Prevention of Postoperative Elevated IOP Associated with Laser Surgery 1.4 Induction of Miosis
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Instill one drop in the eye(s) up to four times daily ( 2 ). 2.1 Reduction of Elevated Intraocular Pressure (IOP) in Patients with Open-Angle Glaucoma or Ocular Hypertension One drop of pilocarpine hydrochloride ophthalmic solution 1%, 2% or 4% should be applied topically in the eye(s) up to four times daily. Pilocarpine-naïve patients should be started on the 1% concentration as higher concentrations are often not tolerated initially. The frequency of instillation and concentration of pilocarpine hydrochloride ophthalmic solution are determined by the severity of the elevated intraocular pressure and miotic response of the patient. To limit systemic exposure to pilocarpine, patients may be instructed to perform punctal occlusion for 2 minutes after instillation of pilocarpine hydrochloride ophthalmic solution. 2.2 Management of Acute Angle-Closure Glaucoma Prior to pilocarpine hydrochloride ophthalmic solution use, treatment with secretory suppressants and hyperosmotic agents may be needed to lower IOP below 50 mmHg and relieve iris ischemia. For initial management of acute angle-closure glaucoma, one drop of pilocarpine hydrochloride ophthalmic solution 1% or 2% may be applied topically in the eye(s) up to three times over a 30-minute period. If laser iridoplasty or iridomy is used to break the attack, one drop of pilocarpine hydrochloride ophthalmic solution 4% should be administered prior to the procedure. Following laser iridoplasty, one drop of pilocarpine hydrochloride ophthalmic solution 1% should be administered four times daily until an iridotomy can be performed. 2.3 Prevention of Postoperative Elevated IOP Associated with Laser Surgery One drop of pilocarpine hydrochloride ophthalmic solution 1%, 2% or 4% (or two drops administered five minutes apart) should be applied topically in the eye(s) 15 to 60 minutes prior to surgery. 2.4 Induction of Miosis One drop of pilocarpine hydrochloride ophthalmic solution 1%, 2% or 4% (or two drops administered five minutes apart) should be applied topically in the eye(s). 2.5 Use with Other Topical Ophthalmic Medications Pilocarpine hydrochloride ophthalmic solution may be used in combination with beta-blockers, carbonic anhydrase inhibitors, sympathomimetics or hyperosmotic agents. If more than one topical ophthalmic drug is being used, the drugs should be administered at least five ( 5 ) minutes apart. 2.6 Use in Pediatric Patients In children under 2 years of age, one drop of pilocarpine hydrochloride ophthalmic solution 1% should be applied topically in the eye(s) three times daily. Children 2 years of age and over should be dosed as for adults. For the induction of miosis prior to goniotomy or trabeculotomy in children, one drop of pilocarpine hydrochloride ophthalmic solution 1% or 2% should be applied topically in the eye 15 to 60 minutes prior to surgery.
