Drug Catalog - Product Detail
PHENYTOIN SODIUM ER CP 100MG 1000
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 62756-0402-03 | SUN PHARMACEUTICALS | 1000 | 100MG | CAPSULE |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
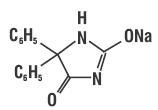
11 DESCRIPTION Phenytoin sodium is related to the barbiturates in chemical structure, but has a five membered ring. The chemical name is sodium 5,5-diphenyl-2,4 imidazolidinedione, having the following structural formula: Each 100 mg extended phenytoin sodium capsule for oral administration contains 100 mg phenytoin sodium, USP. Also contains lactitol monohydrate, sodium lauryl sulfate, talc and magnesium stearate. The capsule shell contains gelatin, and black printing ink, which contains black iron oxide, FD&C Blue No. 2, FD&C Red No. 40, FD&C Blue No. 1, D&C Yellow No. 10, shellac glaze and SDA 3A alcohol or N-butyl alcohol and propylene glycol. Product in vivo performance is characterized by a slow and extended rate of absorption with peak blood concentrations expected in 4 to 12 hours as contrasted to Prompt Phenytoin Sodium Capsules, USP with a rapid rate of absorption with peak blood concentration expected in 1½ to 3 hours. spl-phenytoin-structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied Extended Phenytoin Sodium Capsules USP, 100 mg are supplied as follows: Transparent # 3 capsule, filled with white to off-white powder, with the code '402' imprinted on the cap and body. Bottles of 100: NDC 62756-402-01 Bottles of 100 Non Child Resistant Cap: NDC 62756-402-02 Bottles of 1000 Non Child Resistant Cap: NDC 62756-402-03 16.2 Storage and Handling Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]. Protect from light and moisture. Dispense in a tight, light-resistant container as defined in the USP.
Indications & Usage
1 INDICATIONS & USAGE Extended phenytoin sodium capsules are indicated for the treatment of tonic-clonic (grand mal) and psychomotor (temporal lobe) seizures and prevention and treatment of seizures occurring during or following neurosurgery. Extended phenytoin sodium capsules are indicated for the treatment of tonic-clonic (grand mal) and psychomotor (temporal lobe) seizures and prevention and treatment of seizures occurring during or following neurosurgery. (1)
Dosage and Administration
2 DOSAGE & ADMINISTRATION 2.1 Adult Dosage Divided daily dosage: The recommended starting dose for adult patients who have received no previous treatment is one 100 mg extended phenytoin sodium capsules by mouth three times daily. Adjust the dosage to suit individual requirements up to a maximum of two capsules three times a day. For most adults, the satisfactory maintenance dosage will be one capsule three to four times a day. Once-a-day dosage: In adults, if seizure control is established with divided doses of three 100 mg extended phenytoin sodium capsules daily, once-a-day dosage with 300 mg of extended phenytoin sodium capsules may be considered. Studies comparing divided doses of 300 mg with a single daily dose of this quantity indicated absorption, peak serum levels, biologic half-life, difference between peak and minimum values, and urinary recovery were equivalent. Once-a-day dosage offers a convenience to the individual patient or to nursing personnel for institutionalized patients and is intended to be used only for patients requiring this amount of drug daily. A major problem in motivating noncompliant patients may also be lessened when the patient can take this drug once a day. However, patients should be cautioned not to miss a dose, inadvertently. Only extended phenytoin sodium capsules are recommended for once-a-day dosing. Inherent differences in dissolution characteristics and resultant absorption rates of phenytoin due to different manufacturing procedures and/or dosage forms preclude such recommendation for other phenytoin products. When a change in the dosage form or brand is prescribed, careful monitoring of phenytoin serum levels should be carried out. Loading dose: Some authorities have advocated use of an oral loading dose of phenytoin in adults who require rapid steady-state serum levels and where intravenous administration is not desirable. This dosing regimen should be reserved for patients in a clinic or hospital setting where phenytoin serum levels can be closely monitored. Patients with a history of renal or liver disease should not receive the oral loading regimen. Initially, one gram of extended phenytoin sodium capsules are divided into three doses (400 mg, 300 mg, 300 mg) and administered at two-hour intervals. Normal maintenance dosage is then instituted 24 hours after the loading dose, with frequent serum level determinations. 2.2 Pediatric Dosage The recommended starting dosage for pediatric patients is 5 mg/kg/day by mouth in two or three equally divided doses, with subsequent dosage individualized to a maximum of 300 mg daily in divided doses. A recommended daily maintenance dosage is usually 4 to 8 mg/kg/day in equally divided doses. Children over 6 years and adolescents may require the minimum adult dosage (300 mg/day). 2.3 Dosage Adjustments Dosage should be individualized to provide maximum benefit. In some cases, serum blood level determinations may be necessary for optimal dosage adjustments. Trough levels provide information about clinically effective serum level range and confirm patient compliance, and are obtained just prior to the patient’s next scheduled dose. Peak levels indicate an individual’s threshold for emergence of dose-related side effects and are obtained at the time of expected peak concentration. Therapeutic effect without clinical signs of toxicity occurs more often with serum total concentrations between 10 and 20 mcg/mL (unbound phenytoin concentrations between 1 and 2 mcg/mL), although some mild cases of tonic-clonic (grand mal) epilepsy may be controlled with lower serum levels of phenytoin. In patients with renal or hepatic disease, or in those with hypoalbuminemia, the monitoring of unbound phenytoin concentrations may be more relevant [see Dosage and Administration (2.5)] . With recommended dosage, a period of seven to ten days may be required to achieve steady-state blood levels with phenytoin and changes in dosage (increase or decrease) should not be carried out at intervals shorter than seven to ten days. 2.4 Switching Between Phenytoin Formulations The free acid form of phenytoin is used in phenytoin-125 suspension and phenytoin chewable tablets. Extended phenytoin sodium capsules and parental phenytoin are formulated with the sodium salt of phenytoin. Because there is approximately an 8% increase in drug content with the free acid form over that of the sodium salt, dosage adjustments and serum level monitoring may be necessary when switching from a product formulated with the free acid to a product formulated with the sodium salt and vice versa. 2.5 Dosing in Patients with Renal or Hepatic Impairment or Hypoalbuminemia Because the fraction of unbound phenytoin is increased in patients with renal or hepatic disease, or in those with hypoalbuminemia, the monitoring of phenytoin serum levels should be based on the unbound fraction in those patients [see Warnings and Precautions (5.9) and Use in Specific Populations (8.6)]. 2.6 Geriatric Dosage Phenytoin clearance is decreased slightly in elderly patients and lower or less frequent dosing may be required [see Clinical Pharmacology (12.3)]. 2.7 Dosing during Pregnancy Decreased serum concentrations of phenytoin may occur during pregnancy because of altered phenytoin pharmacokinetics. Periodic measurement of serum phenytoin concentrations should be performed during pregnancy, and the extended phenytoin sodium capsules dosage should be adjusted as necessary. Postpartum restoration of the original dosage will probably be indicated [see Use in Specific Populations (8.1)]. Because of potential changes in protein binding during pregnancy, the monitoring of phenytoin serum levels should be based on the unbound fraction. Adult starting dose in patients who have received no previous treatment is one 100 mg extended phenytoin sodium capsule three times a day, with dose adjustments as necessary. For most adults, the satisfactory maintenance dose will be one capsule three to four times a day. An increase, up to two capsules three times a day may be made, if necessary. (2.1) Adult once-a-day dose: If seizure control is established with divided doses of three 100 mg extended phenytoin sodium capsules daily, once-a-day dosage with 300 mg extended phenytoin sodium capsules may be considered. (2.1) Adult loading dose: reserved for patients in a clinic or hospital setting who require rapid steady-state serum levels and where intravenous administration is not desired. Refer to full prescribing information. (2.1) Pediatric starting dose is 5 mg/kg/day in two to three equally divided doses, with dosage adjustments as necessary, up to a maximum of 300 mg daily. Maintenance dosage is 4 to 8 mg/kg/day. (2.2) Serum blood level determinations may be necessary for optimal dosage adjustments—the clinically effective serum total concentration is 10 to 20 mcg/mL (unbound phenytoin concentration is 1 to 2 mcg/mL). (2.3)
