Drug Catalog - Product Detail
PHENELZINE SULFATE TB 15MG 60
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 43386-0360-21 | LUPIN PHARMACEUTICALS | 60 | 15MG | TABLET |
PACKAGE FILES
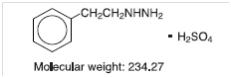

Generic Name
PHENELZINE SULFATE
Substance Name
PHENELZINE SULFATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA200181
Description
DESCRIPTION Phenelzine Sulfate Tablets, USP (phenelzine sulfate) is a potent inhibitor of monoamine oxidase (MAO). Phenelzine sulfate is a hydrazine derivative. It has a molecular weight of 234.27 and is chemically described as C 8 H 12 N 2 • H 2 SO 4 . Its chemical structure is shown below: Each Phenelzine Sulfate Tablets film-coated for oral administration contains phenelzine sulfate equivalent to 15 mg of phenelzine base and the following inactive ingredients: mannitol, USP; colloidal silicon dioxide, NF; povidone, USP; edetate disodium, USP; magnesium stearate, NF; purified water, USP; polyvinyl alcohol part hydrolyzed USP, polyethylene glycol-3350 NF, FD&C yellow # 6, talc USP and titanium dioxide USP. 4e68c327-figure-01
How Supplied
HOW SUPPLIED Each Phenelzine Sulfate Tablets is orange, biconvex, film-coated tablets, debossed with "NL" on one side and "360" on the other side. Contains phenelzine sulfate equivalent to 15 mg of phenelzine base. NDC 43386-360-21. Bottle of 60 Storage Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature] . Preserve in tight containers, protected from heat and light. Rx only Manufactured by: Novel Laboratories Inc, Somerset, NJ 08873 Manufactured for: Lupin Pharmaceuticals, Inc. Baltimore, MD 21202 PI3600000204 Rev. 05/2016
Indications & Usage
INDICATIONS AND USAGE Phenelzine Sulfate Tablets, USP has been found to be effective in depressed patients clinically characterized as "atypical," "nonendogenous," or "neurotic." These patients often have mixed anxiety and depression and phobic or hypochondriacal features. There is less conclusive evidence of its usefulness with severely depressed patients with endogenous features. Phenelzine Sulfate Tablets should rarely be the first antidepressant drug used. Rather, it is more suitable for use with patients who have failed to respond to the drugs more commonly used for these conditions.
Dosage and Administration
DOSAGE AND ADMINISTRATION Initial dose The usual starting dose of Phenelzine Sulfate Tablets is one tablet (15 mg) three times a day. Early phase treatment Dosage should be increased to at least 60 mg per day at a fairly rapid pace consistent with patient tolerance. It may be necessary to increase dosage up to 90 mg per day to obtain sufficient MAO inhibition. Many patients do not show a clinical response until treatment at 60 mg has been continued for at least 4 weeks. Maintenance dose After maximum benefit from Phenelzine Sulfate Tablets is achieved, dosage should be reduced slowly over several weeks. Maintenance dose may be as low as one tablet, 15 mg, a day or every other day, and should be continued for as long as is required.
