Drug Catalog - Product Detail
PERPHENAZINE TAB 8 MG 100CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00591-4103-01 | ACTAVIS PHARMA | 100 | 8MG | TABLET |
PACKAGE FILES




Generic Name
PERPHENAZINE
Substance Name
PERPHENAZINE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA207582
Description
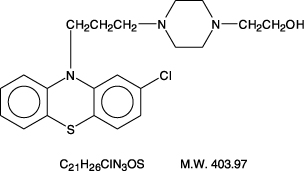
DESCRIPTION Perphenazine (4-[3-(2-chlorophenothiazin-10-yl)propyl]-1-piperazineethanol), a piperazinyl phenothiazine, having the chemical formula, C 21 H 26 CIN 3 OS. It is available as oral tablets containing 2 mg, 4 mg, 8 mg, and 16 mg of perphenazine, USP. Inactive ingredients: lactose (monohydrate), microcrystalline cellulose, magnesium stearate, polyethylene glycol, starch (corn), titanium dioxide, hypromellose and polysorbate 80. Its structural formula is: 1
How Supplied
HOW SUPPLIED Perphenazine tablets USP, 2 mg are white to off white, round biconvex, film-coated tablets, debossed with “ A ” on one side and “ 280 ” on the other side, supplied as: NDC 0591-4101-01 bottles of 100 tablets Perphenazine tablets USP, 4 mg are white to off white, round biconvex, film-coated tablets, debossed with “ A ” on one side and “ 281 ” on the other side, supplied as: NDC 0591-4102-01 bottles of 100 tablets Perphenazine tablets USP, 8 mg are white to off white, round beveled edge biconvex, film-coated tablets, debossed with “ A ” on one side and “ 282 ” on the other side, supplied as: NDC 0591-4103-01 bottles of 100 tablets Perphenazine tablets USP, 16 mg are white to off white, round beveled edge biconvex, film-coated tablets, debossed with “ A ” on one side and “ 283 ” on the other side, supplied as: NDC 0591-4104-01 bottles of 100 tablets Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Protect from light. Dispense in a tight, light-resistant container.
Indications & Usage
INDICATIONS AND USAGE Perphenazine is indicated for use in the treatment of schizophrenia and for the control of severe nausea and vomiting in adults. Perphenazine has not been shown effective for the management of behavioral complications in patients with mental retardation.
Dosage and Administration
DOSAGE AND ADMINISTRATION Dosage must be individualized and adjusted according to the severity of the condition and the response obtained. As with all potent drugs, the best dose is the lowest dose that will produce the desired clinical effect. Since extrapyramidal symptoms increase in frequency and severity with increased dosage, it is important to employ the lowest effective dose. These symptoms have disappeared upon reduction of dosage, withdrawal of the drug, or administration of an antiparkinsonian agent. Prolonged administration of doses exceeding 24 mg daily should be reserved for hospitalized patients or patients under continued observation for early detection and management of adverse reactions. An antiparkinsonian agent, such as trihexyphenidyl hydrochloride or benztropine mesylate, is valuable in controlling drug-induced extrapyramidal symptoms. Suggested dosages for various conditions follow: Moderately disturbed nonhospitalized patients with schizophrenia 4 to 8 mg three times a day initially; reduce as soon as possible to minimum effective dosage. Hospitalized patients with schizophrenia 8 to 16 mg two times a day to four times a day; avoid dosages in excess of 64 mg daily. Severe nausea and vomiting in adults 8 to 16 mg daily in divided doses; 24 mg occasionally may be necessary; early dosage reduction is desirable. Elderly Patients With increasing age, plasma concentrations of perphenazine per daily ingested dose increase. Geriatric dosages of perphenazine preparations have not been established, but initiation of lower dosages is recommended. Optimal clinical effect or benefit may require lower doses for a longer duration. Dosing of perphenazine may occur before bedtime, if required.
