Drug Catalog - Product Detail
OXYBUTYNIN CHLORIDE ER TABS 10MG 500CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65162-0372-50 | AMNEAL PHARMACEUTICALS | 500 | 10MG | TABLET |
PACKAGE FILES
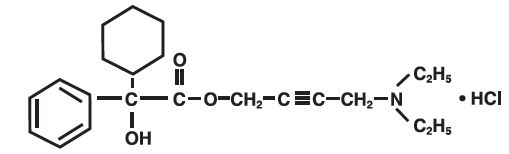


Generic Name
OXYBUTYNIN CHLORIDE
Substance Name
OXYBUTYNIN CHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA204010
Description
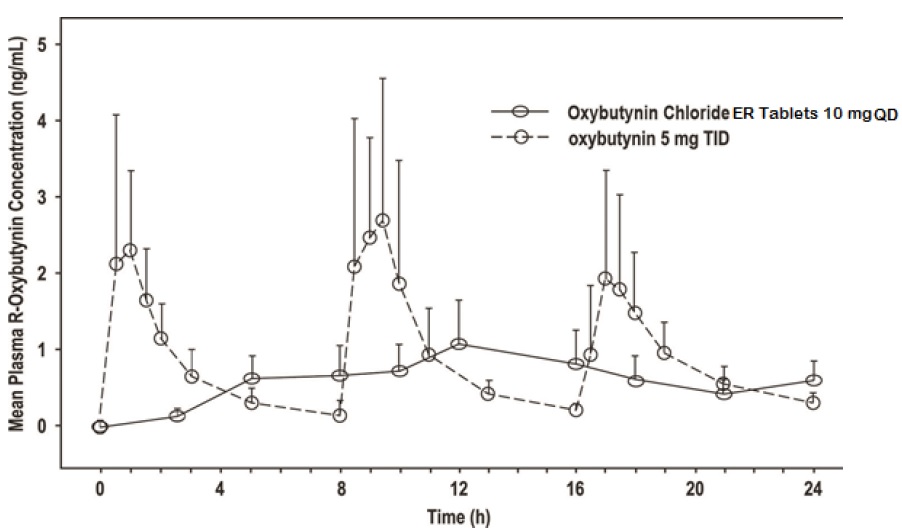
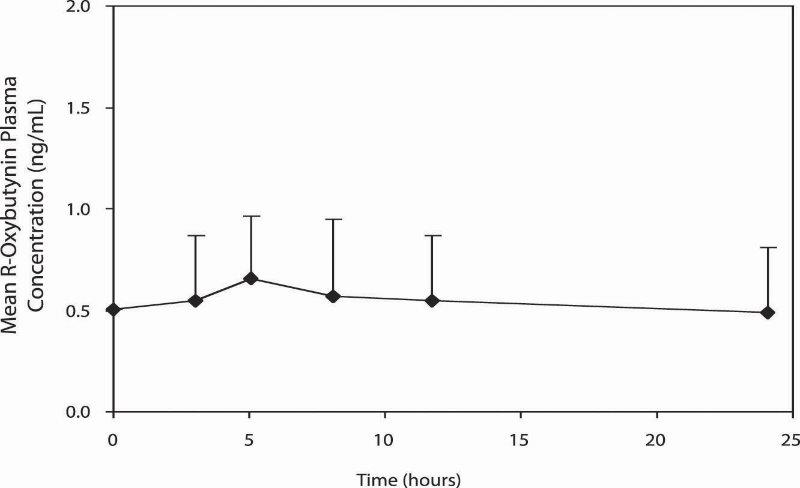
11 DESCRIPTION Oxybutynin chloride, USP is an antispasmodic, muscarinic antagonist. Each oxybutynin chloride extended-release tablet, USP contains 5 mg, 10 mg, or 15 mg of oxybutynin chloride USP, formulated as a once-a-day controlled-release tablet for oral administration. Oxybutynin chloride, USP is administered as a racemate of R- and S-enantiomers. Chemically, oxybutynin chloride, USP is d,l (racemic) 4-diethylamino-2-butynyl phenylcyclohexylglycolate hydrochloride. The empirical formula of oxybutynin chloride, USP is C 22 H 31 NO 3 •HCl. Its structural formula is: Oxybutynin chloride, USP is a white crystalline solid with a molecular weight of 393.9. It is readily soluble in water and acids, but relatively insoluble in alkalis. Oxybutynin chloride extended-release tablets, USP also contains the following inert ingredients: hydrogenated vegetable oil, hypromellose, isopropyl alcohol, lactose monohydrate, methacrylic acid copolymer, microcrystalline cellulose, polysorbate 80, sodium lauryl sulfate, talc and triethyl citrate. The 5 mg tablets also contain D&C Red #27 and FD&C Blue #1. The 10 mg tablets also contain FD&C Red #40 and FD&C Yellow #6. Meets USP Dissolution Test 7. System Components and Performance Oxybutynin chloride extended-release tablet, USP uses a pH-independent hydrophilic matrix and pH dependent enteric coating to deliver oxybutynin chloride, USP at a controlled rate over approximately 24 hours. The oxybutynin chloride extended-release tablet, USP comprises of an inner core made of the drug, rate controlling pH independent polymer and other excipients. The tablet core is then coated with pH dependent enteric coating polymer. When tablets will be exposed to an acidic environment such as the stomach, the drug release will be minimal due to outer enteric coating of the pH dependent polymer. The outer enteric coating upon reaching an environment of pH 5.5 and above will start to dissolve and the polymer in the inner core tablet will hydrate to form a gel layer and the drug releases via diffusion process. 1
How Supplied
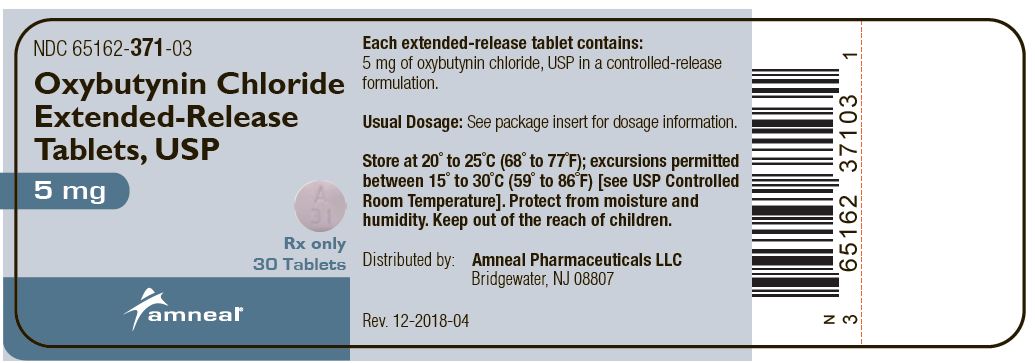
16 HOW SUPPLIED/STORAGE AND HANDLING Oxybutynin chloride extended-release tablets USP, 5 mg are supplied as purple, round, biconvex, coated tablets debossed with “A31” on one side and plain on the other side. They are available as follows: Bottles of 30: NDC 65162-371-03 Bottles of 90: NDC 65162-371-09 Bottles of 100: NDC 65162-371-10 Bottles of 500: NDC 65162-371-50 Oxybutynin chloride extended-release tablets USP, 10 mg are supplied as pink, round, biconvex, coated tablets debossed with “A32” on one side and plain on the other side. They are available as follows: Bottles of 30: NDC 65162-372-03 Bottles of 90: NDC 65162-372-09 Bottles of 100: NDC 65162-372-10 Bottles of 500: NDC 65162-372-50 Oxybutynin chloride extended-release tablets USP, 15 mg are supplied as white, round, biconvex, coated tablets debossed with “A33” on one side and plain on the other side. They are available as follows: Bottles of 30: NDC 65162-373-03 Bottles of 90: NDC 65162-373-09 Bottles of 100: NDC 65162-373-10 Bottles of 250: NDC 65162-373-25 Bottles of 500: NDC 65162-373-50 Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Protect from moisture and humidity. Keep out of the reach of children.
Indications & Usage
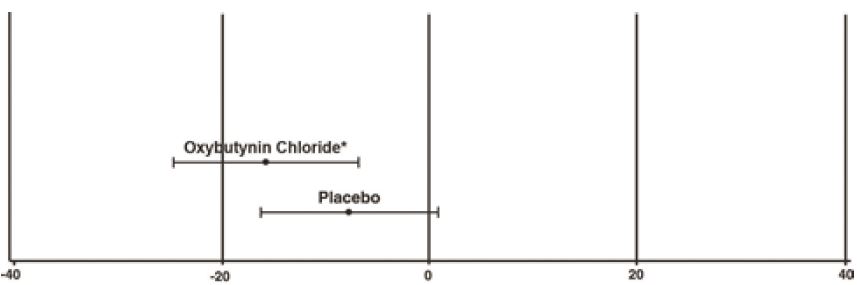
1 INDICATIONS AND USAGE Oxybutynin chloride extended-release tablets are a muscarinic antagonist indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency. Oxybutynin chloride extended-release tablets are also indicated for the treatment of pediatric patients aged 6 years and older with symptoms of detrusor overactivity associated with a neurological condition (e.g., spina bifida). Oxybutynin chloride extended-release tablets are a muscarinic antagonist indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency. (1) Oxybutynin chloride extended-release tablets are also indicated for the treatment of pediatric patients aged 6 years and older with symptoms of detrusor overactivity associated with a neurological condition (e.g., spina bifida). (1)
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Oxybutynin chloride extended-release tablets must be swallowed whole with the aid of liquids, and must not be chewed, divided, or crushed. Oxybutynin chloride extended-release tablets may be administered with or without food. Oxybutynin chloride extended-release tablets must be swallowed whole with the aid of liquids, and must not be chewed, divided, or crushed. Oxybutynin chloride extended-release tablets may be administered with or without food. (2) Adults: Start with 5 mg or 10 mg, once daily at approximately the same time every day. Dose should not exceed 30 mg per day. (2.1) Pediatric patients (6 years of age or older): Start with 5 mg, once daily at approximately the same time every day. Dose should not exceed 20 mg per day. (2.2) 2.1 Adults The recommended starting dose of oxybutynin chloride extended-release tablets is 5 or 10 mg once daily at approximately the same time each day. Dosage may be adjusted in 5-mg increments to achieve a balance of efficacy and tolerability (up to a maximum of 30 mg/day). In general, dosage adjustment may proceed at approximately weekly intervals. 2.2 Pediatric Patients Aged 6 Years of Age and Older The recommended starting dose of oxybutynin chloride extended-release tablets is 5 mg once daily at approximately the same time each day. Dosage may be adjusted in 5-mg increments to achieve a balance of efficacy and tolerability (up to a maximum of 20 mg/day).
